25,000 doses of vaccine for Corona were produced at the Biological Institute • It will first be tested whether the vaccine causes side effects, and what the level of antibodies is in the blood • Israel"
Vaccine for corona
Photography:
AP
The clinical trials in humans on the Israeli vaccine for the Corona of the Biological Institute in Ness Ziona will begin next Sunday, November 1, Defense Minister Bnei Gantz announced tonight (Sunday).
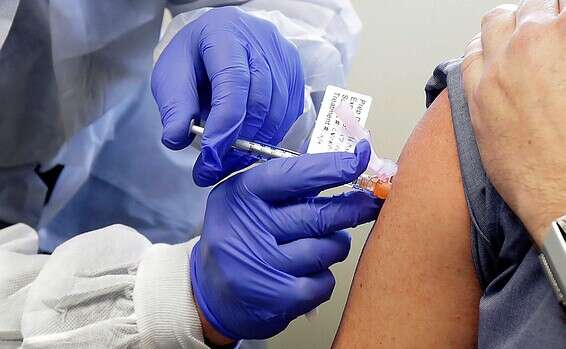
The human clinical trial phase will last several months and will include three phases: Phase 1: First safety tests on 80 healthy volunteers aged 18-55, at Sheba and Hadassah hospitals.
The trial is expected on November 1, initially for two volunteers and depending on their responses to the vaccine will be gradually expanded to 80 people (40 in each medical center).
Each volunteer will receive an injection (vaccine or placebo - dummy).
After a few hours of supervision he will be released to his home for further follow-up of about three weeks.
During the trial, the experimenters will examine whether any side effects have occurred and differentiate whether antibodies to the virus have appeared, indicating a response to the corona virus in the volunteers who received the vaccine.
Phase 2: Extensive safety tests on 960 healthy volunteers aged 18 and over.
The trial is expected to begin in December.
It will be held in parallel at a number of medical centers around the country and is designed to complete vaccine safety tests, pinpoint the right doses and continue to test measures of its effectiveness.
Phase 3: A large-scale trial to test the effectiveness of the vaccine with the participation of up to 30,000 volunteers.
The experiment is scheduled to begin in April-May (subject to the success of the first two phases).
This is the last stage, after which it will be successfully completed, the vaccine can be approved and the entire population vaccinated.
Defense Minister Bnei Gantz welcomed the approval and said: "This is a day of hope for the citizens of Israel, and the researchers at the Biological Institute are fighters of hope. Only two months ago I received the first bottle of the vaccine, and today we already have 25,000 vaccine doses, on the way to the next stage I would like to thank the dozens of researchers who work day and night on this national mission in full cooperation with the Ministry of Health - in this complex period, you are the patrol that paves the way for the citizens of Israel. You have taken on a mission on an international and historical scale. "I, the entire defense establishment, and the entire Israeli government, will continue to give you the support and means necessary to reach a safe and effective blue-and-white vaccine."