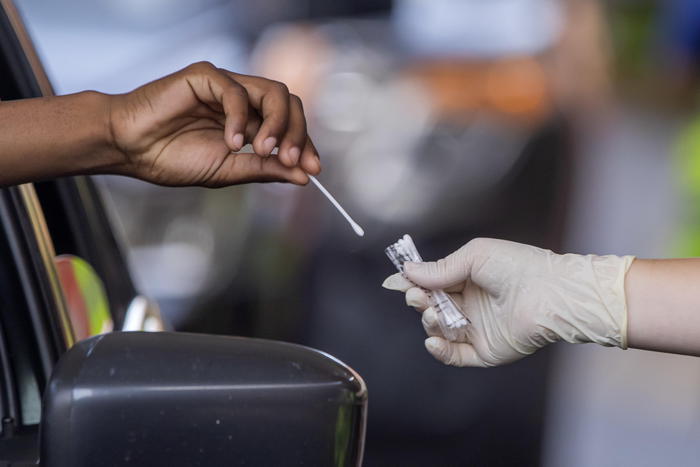
(ANSA) - NEW YORK, NOVEMBER 18 - The Food and Drug Administration (Fda), the American federal agency that deals with medicines, has authorized the first quick do-it-yourself test for coronavirus, which can be used at home autonomously and gives results in 30 minutes.
The emergency approval concerns a kit produced by the Californian company Lucira Health, which will allow potentially infected people not to have to go to hospital or health centers to carry out the swab, with the risk of new infections.
The test, as reported by the US media, can be used by anyone who is at least 14 years old and will cost less than $ 50, but will require a doctor's prescription: initially it will be available through Sutter Health, in Northern California, and Cleveland Clinic Florida, in Miami. Ft.
Lauderdale.
By spring 2021, however, it will be available nationwide through health care operators.
According to the Lucira instructions for use, the test was able to correctly identify 94% of positive and 98% of negative samples diagnosed using a more sensitive laboratory test.
(HANDLE).