From the first days of the pandemic, the urgency to treat the sick led doctors to use drugs designed for other ailments in the hope that they could also work against the disease caused by the new coronavirus, SARS-CoV- two.
The largest study to date on four of these treatments has just shown that none of them save the lives of covid patients, the World Health Organization (WHO) announced Thursday night.
The United Nations agency Solidarity trial was testing the efficacy against SARS-CoV-2 of the antimalarials chloroquine and hydroxychloroquine, the antiviral remdesivir, and the antiretrovirals lopinavir and ritonavirs, used against HIV, and interferon.
None of these drugs have shown significant effects in reducing patient mortality after 28 days of treatment, the WHO has confirmed in a statement.
Solidarity has been a one-of-a-kind patient trial in both its size and the speed with which it was conducted.
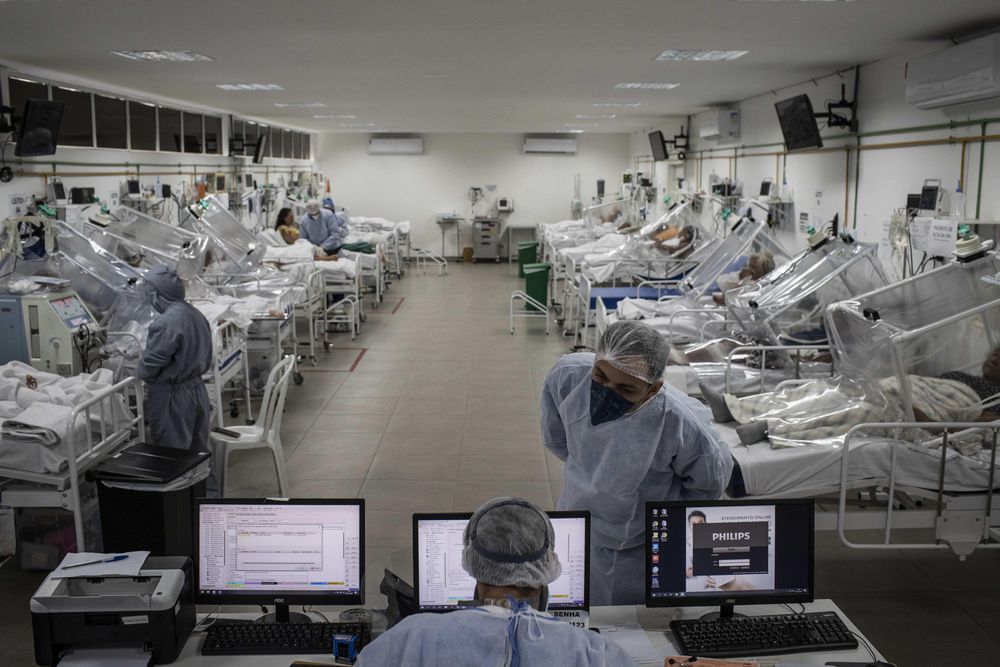
The evolution of more than 11,200 patients in 400 hospitals in 32 countries around the world has been analyzed.
Patients were randomly assigned to receive either only normal care or the same plus one of the aforementioned treatments.
"None of the drugs studied reduced mortality in any subgroup of patients or had effects on the initiation of artificial respiration or the duration of hospital admission," explains the study on the trial, which has been published openly on the web and is being revised to appear in a specialized medical publication, according to the WHO.
These results are the last nail in the coffin for treatments that have been praised by politicians without evidence of their efficacy, especially chloroquine and its derivative hydroxychloroquine.
These drugs described as "revolutionary" by leaders like Donald Trump in the early moments of the pandemic have turned out to have no positive effect and may even be counterproductive in many patients.
The definitive results of Solidarity show not only that there is no effectiveness, but that there are slightly more deaths in the group of patients who received these drugs than in those who did not take them
The WHO already had to stop the use of these drugs in the trial in June on suspicion of increasing mortality, although it later resumed after a more detailed review, as the worrying data came from a study that turned out to be a fraud.
A month later, the organization again removed these two drugs from the trial based on their own data and now the final results show that there is no effectiveness.
In the case of remdesivir, an antiviral developed against Ebola by the American pharmaceutical company Gilead, the results open an important unknown, since the drug has been approved for use in both the United States and Europe temporarily until there are solid data on its effectiveness.
The WHO trial shows that in global terms this drug is not effective.
But a study published last week in the prestigious medical journal
NEJM
pointed out that this drug makes patients recover about five days earlier than those who do not take it.
In that study —which analyzed 1,062 patients— a reduction in mortality was detected in a small group of patients: those who have just begun to receive oxygen but are not yet in serious condition or require assisted ventilation.
Gilead Pharmaceuticals is conducting another clinical trial to test whether remdesivir given with a rheumatoid arthritis drug called baricitinib increases the positive effects seen.
"For all the doctors who are fighting COVID, this is very bad news," sums up José Ramón Arribas, head of infectious diseases at Hospital La Paz in Madrid, who participates in both Solidarity and the Gilead-sponsored remdesivir trials.
“We have to wait for this new WHO study to be reviewed by independent experts and published in a medical journal, but if the data is maintained, it is likely that the regulatory agencies [for drugs, such as the AEMPS in Spain] will release remdesivir of the treatment guidelines in a few days or weeks ”, he explains.
Remdesivir exemplifies how difficult it is to develop an effective drug and show that it works.
Part of the discrepancies between the studies may be due to the design of the studies.
“This antiviral had shown efficacy in in vitro [laboratory] cultures, in animals, and even in humans on a preliminary basis.
The study published last week was scientifically of higher quality than the WHO one, but in return Solidarity has many more patients and also includes a meta-analysis of all the trials published on this drug;
and confirms that it does not have any benefit, "says Arribas.
Roger Paredes, a doctor at the German Trias i Pujol hospital in Barcelona who participates in the Gilead trials, thinks that it is too early to discard remdesivir.
"This drug works if we give it to the people we have to give it to," he explains.
With these results, the only drugs that have shown effectiveness against covid are dexamethasone and other corticosteroids, which do reduce the mortality of patients in serious condition.
The WHO explains that it is studying the possibility of testing new antivirals, immunomodulators and monoclonal antibodies in the hospitals in the Solidarity study.
You can follow
MATERIA
on
,
,
or subscribe here to our
newsletter
.




/cloudfront-eu-central-1.images.arcpublishing.com/prisa/BS6KLR7HWVHDBPE2NL7LDTVNKE.jpg)









