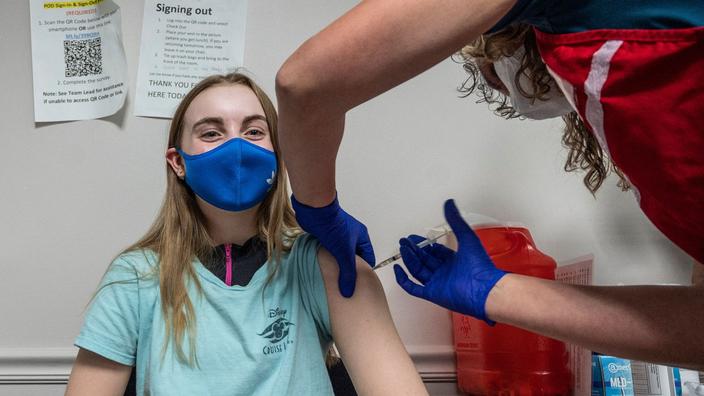
The European Medicines Agency (EMA) will vote on Friday on the authorization of Pfizer / BioNTech's vaccine for 12-15 year olds, the European regulator said.
To read also: Vaccines: these young people who circumvent the rules to "find a normal life"
If approved, the serum will be the first vaccine to get the green light from the EMA to be administered to young people in the 27 countries of the European Union.
For the moment, its authorization is limited to people over 16 years old.
The EMA will hold a press conference on Friday to share the "
results of an extraordinary meeting of its Committee for Medicinal Products for Human Use (...) regarding the pediatric indications of the Comirnaty vaccine
," the agency said on Wednesday in a report. communicated.
Comirnaty is the trade name of the anti-Covid 19 vaccine developed by the American pharmaceutical giant Pfizer and the German laboratory BioNTech.
The American Medicines Agency (FDA) has already cleared the Pfizer vaccine for 12-15 year olds.
EMA Executive Director Emer Cooke announced in early May that the Amsterdam-based regulator would conduct an accelerated assessment of data submitted by vaccine developers for authorization to 12-15 year olds. The results of this were initially expected in June. Emer Cooke told European newspapers on May 11 that the EMA had received the data from Pfizer / BioNTech and that the agency - which was also awaiting "
data from clinical trials and the study conducted in Canada
" - was planning "
To speed up
" its evaluation.