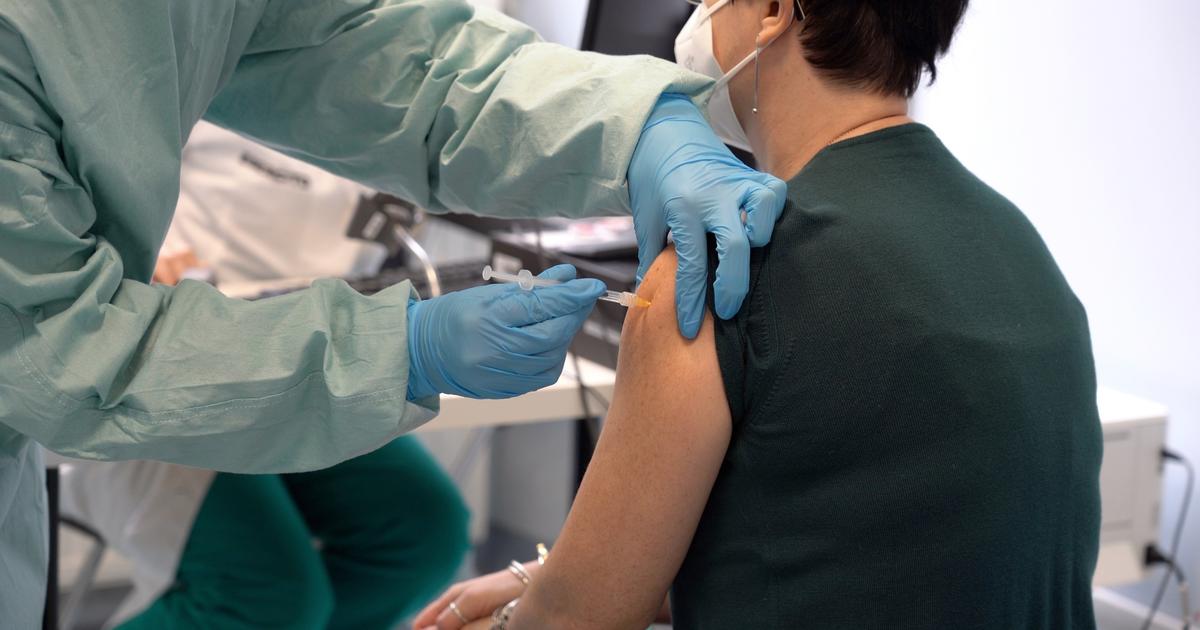
The French giant has undeniably missed its sprint.
What will happen to the long-distance race to produce its own vaccines against Covid-19?
Those of its competitors, the Americans Pfizer-BioNTech and Moderna, are already on the market.
And the Swedish-British AstraZeneca hopes to obtain its authorization this month… The two Sanofi vaccines - one said to be recombinant protein;
the other to messenger RNA, on the model of those of Pfizer-BioNTech and Moderna - they are not expected until the end of 2021. Suffice to say an eternity, at a time when the global and French demand for the proposed solutions does not does not finish growing.
Dosage error ...
Last spring, then fully in the race, the world number 1 in sales of influenza vaccines seems to make a consistent choice.
“Sanofi started with strong skills with its recombinant protein vaccine candidate,” explains Nathalie Coutinet, health economist.
The group can indeed rely on solid data: it has tested this method with its latest influenza vaccine, authorized since 2017 in the United States.
Ultimately, the formula must be sold for less than 10 euros.
But patatras!
The test phase reveals an insufficient concentration of antigen in the vaccine, and by extension an unsatisfactory immune response, especially in adults over 50 years old.
How to explain this lack of dosage?
The "reagents [
Editor's note: control, for antigen measurements
] used were not of sufficient quality or purity", explained Thomas Triomphe, vice-president of the "vaccines" branch, at " Wall Street Journal ”.
Erratic acquisition strategy for “biotechs”.
The other vaccine candidate, the one known as messenger RNA, is hardly more advanced.
To understand why, this time we have to go further back in time.
“Sanofi missed the biotech revolution [
Editor's note start-ups specializing in health innovation
], confides a senior official at the Directorate-General for Enterprises.
They have not staked enough in its companies for their basic research.
Or so wrong.
"" Wrong!
»Retorts at Sanofi, stressing that the collaboration agreement with the American biotech Translate Bio, the basis of the work in progress on the messenger RNA vaccine, dates from 2018.
The "bad luck effect".
Acquiring a biotech, of course, but you still have to find the right one.
Jackpot for Pfizer with BioNtech, as Sanofi continues its work.
"Without doubt there is also a bad luck effect", admits the health economist Nathalie Coutinet.
Knowing that the discovery of a vaccine would cost on average at least one billion euros.
“Result, number one on certain vaccines, we find ourselves last in the class, regrets Morad Zerrouali, central CGT union manager of the Pharmacy branch.
And all our talents go to the competition.
The brain drain is our problem.
"
READ ALSO>
400 research and development jobs eliminated: Sanofi's troubling strategy
Additional snub, the government urged the French giant to make its production lines available.
To be, in a way, a simple subcontractor to its competitors who have already developed a vaccine.
“We asked them if they did not have mobilized capacities to boost the manufacture of existing vaccines,” explains Agnès Pannier-Runacher, the Minister in charge of Industry.
"We watch, but it takes time to change lines," we explained on Monday at Sanofi.
Another problem - perhaps more profound: "It is technically feasible, but it poses an important patent problem, while Sanofi is developing its own messenger RNA vaccine solution", points out an expert in the sector.