The Federal Food and Drug Administration (FDA) on Wednesday authorized the booster dose of Pfizer-BioNTech for people 65 and older and those who are at high risk for serious illness
at least six months after their second dose.
Those who may suffer a severe coronavirus infection or who are at high risk of contagion from being more exposed to the virus are considered to be at high risk.
[We answer 5 key questions about the coronavirus vaccine booster]
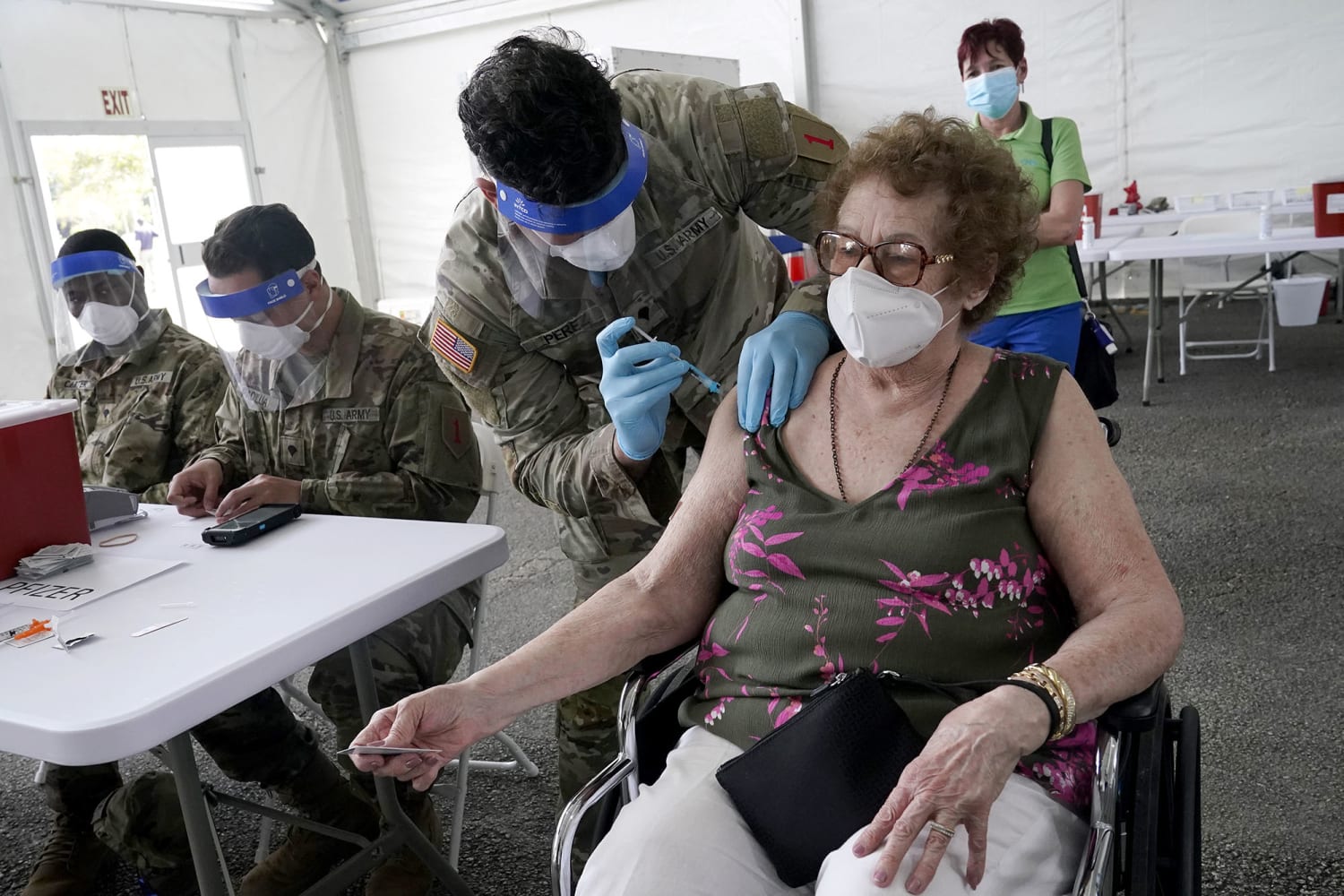
Loida Méndez, 86, receives the first dose of Pfizer's COVID-19 vaccine from US Army physician Luis Pérez, at a FEMA vaccination center at Miami Dade College in North Miami, Florida.Marta Lavandier / AP
The authorization leads to what will likely be a nationwide campaign to administer vaccines, starting with the most vulnerable population groups.
This can allow tens of millions of already immunized people to receive boosters at pharmacies, health clinics, doctor's offices, and other places.
The agency's decision comes days after an FDA panel of experts made a similar recommendation on the need for a booster dose to the most vulnerable, but
not to the general population
.
More information shortly ...