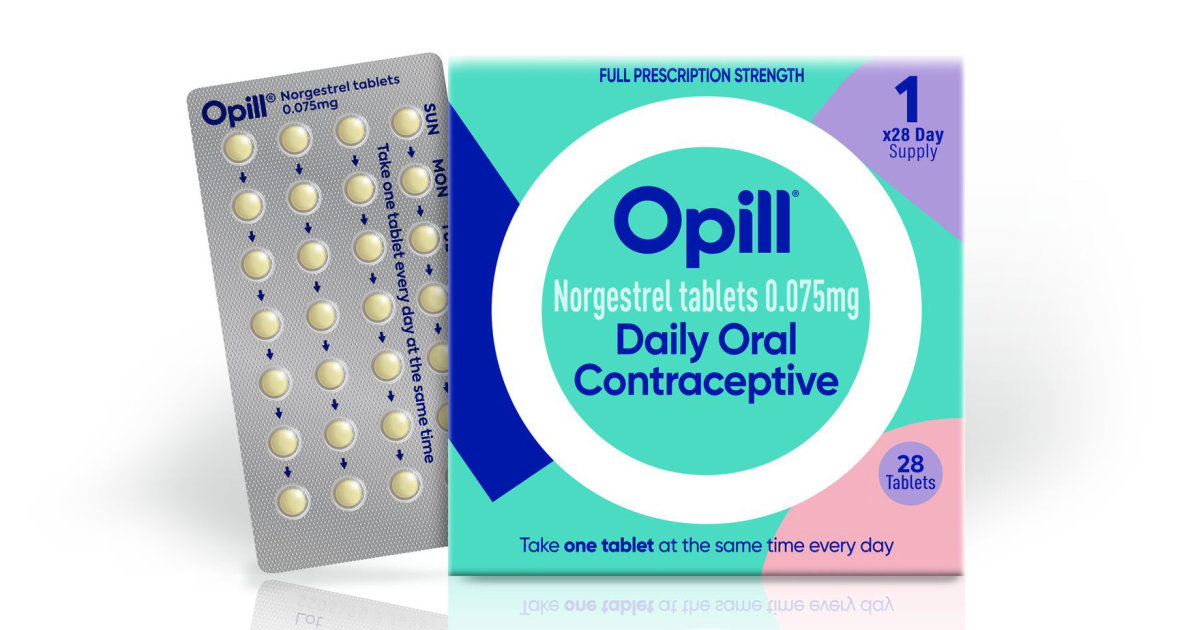
The agency's board members voted unanimously to make the pill available over-the-counter, saying the benefits outweigh the risks. The FDA is not required to follow the committees' recommendation, though the vote is expected to weigh heavily in its final decision, expected by the end of the summer.
If approved, Opill, from French drugmaker HRA Pharma, would be the first over- the-counter birth control pill in the United States. It contains a hormone, progestin, and is taken daily.