By presenting his deconfinement plan, the Prime Minister, Edouard Philippe, confirmed that France was ready to massively test the population from May 11, in particular thanks to serological tests. But today, the vagueness remains on this process.
What are serological tests for?
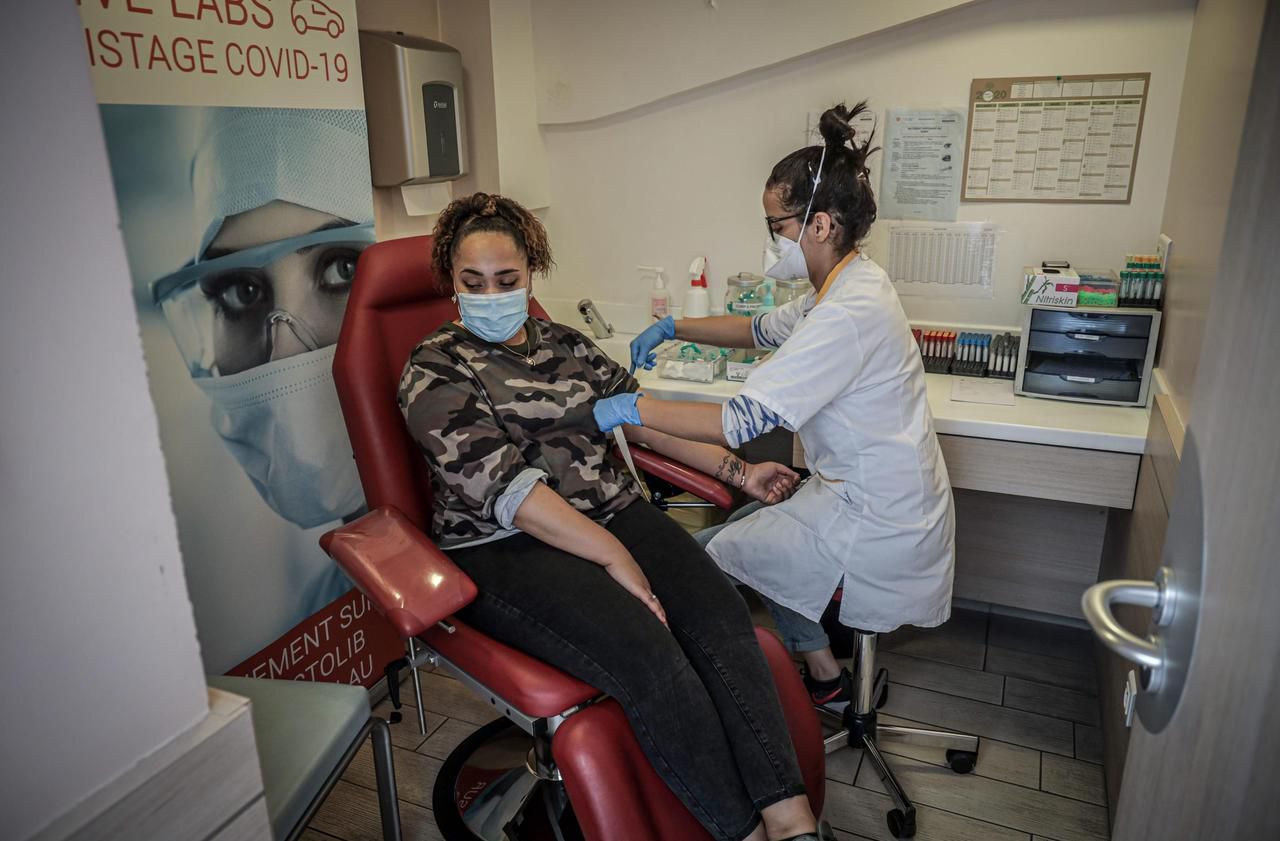
Unlike virological screening, which allows you to know if you have the virus when you are tested, serological tests consist in looking for traces of antibodies in the blood, proof that you have caught Covid, even without symptoms, and that the body defended itself. They appear several days after infection.
There are several families of tests. The first, called Elisa, consists of a blood test in the city laboratory. The blood is centrifuged, rid of red and white blood cells to take an interest in the serum, where the antibodies are present.
A second, a portable test, ultra fast, and analyzed by a health professional, aims to deposit a drop of blood collected on the finger, on a case with a strip that changes color according to the result.
Finally, there are self-tests, performed by the patient himself. In the coming days, the High Health Authority (HAS) will issue an opinion on the reliability of the latter two.
Are there many requests?
As the wave of the epidemic passed, everyone wondered, "Did I get the virus? And am I therefore immune? Since deconfinement, it's the rush for labs, where anyone can be tested without a prescription, provided they pay. A manager of an analysis laboratory in Paris notes this: “Many people want to know. In two days, we had to deal with 150 requests. We have had everything from people with or without symptoms, in addition to our staff, who want to be tested. The first results show a rate of 17% positive. "
Industrial Roche has already received a million test orders from labs after a week of marketing. "It is increasing strongly, we can feel the movement coming." NG Biotech, which manufactures tests made from a drop of blood, has already produced 70,000 in April, especially for the army, before rising to 2 million per month by July.
If we have antibodies, does that mean that we are immune?
Therein lies the problem. Today, no one has the answer. "After a serological test, I can say if there is an antibody, but I cannot note in my conclusions that the person is no longer contagious or immune," explains François Blanchecotte, president of the national union of biologists , who works in Tours (Indre-et-Loire).
Newsletter - The essentials of the news
Every morning, the news seen by Le ParisienI'm registering
Your email address is collected by Le Parisien to allow you to receive our news and commercial offers. Find out more
Faced with this new virus, researchers do not know how long, the antibodies protect and wonder if we can be reinfected. "There seem to be cases in China," says the biologist. According to Inserm, they are therefore not an "immunity passport". The risk is that we believe we are protected and that we relax in the face of barrier gestures.
#CORONAVIRUS # COVID19 | 2 types of tests can detect the presence of the virus or antibodies in the body.
➡️ virological diagnostic tests, to detect patients, isolate them and break the chains of contamination
➡️ serological tests
Explanations. pic.twitter.com/SBpJgmq3VL
For this reason, HAS judges that screening the entire population is not "relevant". In late March, the government announced the pre-purchase of 5 million of these tests in the hope of speeding up after May 11, before being more nuanced in late April. They were ultimately excluded from the national deconfinement strategy. "To date, there are too many uncertainties about their reliability," replies the Directorate General of Health.
Are they reliable?
The HAS has set up specifications. Serological tests can be marketed thanks to CE marking upon simple declaration by the manufacturer. Then, the national reference centers for respiratory infection viruses, mandated by the Ministry of Health, are responsible for evaluating them at the Institut Pasteur, in Paris, or at the Hospices Civils de Lyon.
What is the error rate? The proportion of false negatives would relate today to around 5% of the best performing tests, but it could climb to 40% of those of lower quality, had pointed out the Minister of Health, Olivier Véran, on April 19. But there is also the risk of "false positives" which gives an assurance to a person that he has been sick and believes that he is immune when he is not.
"Depending on the results obtained, the diagnostic test can be subject to certification by the French health authorities in order to be reimbursed by social security", replies the Institut Pasteur. But all of them are far from having been. "Forty tests are being evaluated," reacts François Blanchecotte. According to the drug agency, it is expected that the Ministry of Health will make public "a list of serological tests". For the moment, none is yet approved. "But some have nevertheless been the subject of preliminary study by scientific teams", nuance François Blanchecotte.
Who are they recommended for?
The High Health Authority recommends them in certain indications, because these serological tests do allow us to know if we have had the virus. They are useful in research to assess how much of the population has been infected. And also within a specific framework for asymptomatic nursing staff or collective accommodation, such as medico-social establishments, prisons, barracks ... But they are not reimbursed. Health insurance confirms that "these tests are still not supported".
Why this mess?
The tests differ from one industrialist to another. Some detect a group of antibodies in the blood, others only search for "immunoglobulins G", known to be the most protective without any real evidence. Developed in an emergency, in forty-two days for the Roche laboratory - instead of usually two years -, they are marketed before being certified by the authorities. “We know there are good tests, and bad tests, but we don't know which ones. It's a mess. We can not explain this delay, "wonders Lionel Barrand, president of the union of young medical biologists.
In the meantime, the laboratories are sailing on sight, forced to manage the issue directly with their suppliers. “We have chosen a test offered by an industrial company that we know well. We checked with our technicians that it works, and this is indeed the case, explains a manager of a medical analysis laboratory in Paris. But it would be much more reassuring if the approvals had been published before. "
For his part, Dr. Cédric Carbonneil, head of the evaluation service of the High Authority for Health, understands the expectations of professionals. "It is true that it took a little time but we have no decision-making power". The general direction of health assures us, the long awaited list will be published "in the next few days".
VIDEO. Coronavirus: dogs trained to screen for Covid-19