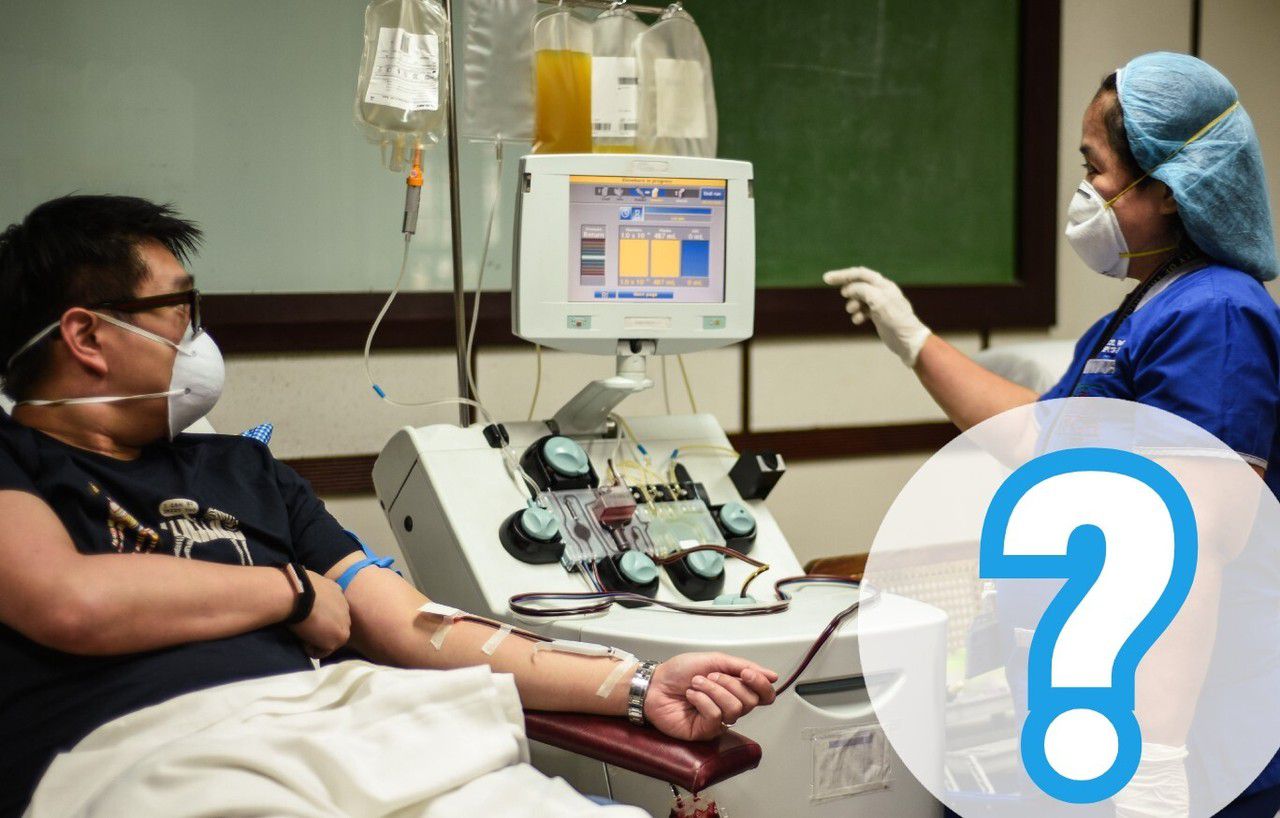
Every day, Le Parisien mobilizes to answer your questions around the coronavirus. Today we are interested in the question of Trinh, who wonders if it is possible to take the antibodies from the healed people to inject them into the sick.
This treatment exists under the name of serotherapy. It is the transfusion of convalescent plasma: a liquid component of the blood of a cured patient that concentrates antibodies after an illness is taken and injected into a patient. The technique has already proven effective in small-scale studies against infectious diseases like Ebola.
Several clinical trials worldwide
For SARS-CoV-2, plasma transfusion is at the heart of several clinical trials worldwide, notably in China, the United States and Austria. In France, the Covisplasm project led by the Assistance Publique-Hôpitaux de Paris (AP-HP), the French Blood Establishment (EFS) and the National Institute of Health and Medical Research (Inserm), has started At the beginning of April.
“The plasma of people who have recovered from Covid-19 contains these antibodies that their bodies have developed. These antibodies could help patients in the acute phase of the disease to fight the virus, "the institutions said in a statement.
Targeted samples have started in Ile-de-France, Grand-Est and Bourgogne-Franche-Comté from around 200 patients who have been cured for at least 14 days. The latter were "personally invited to donate their plasma to the EFS," the statement said.
Expanded outside clinical trials
While the results of these studies are still pending, the National Medicines Agency (ANSM) announced at the end of April that it had opened, "exceptionally", the use of plasma from patients cured outside of these clinical trials, in order to 'Increase the chances of survival' for these patients.
When it is done outside of a study, the treatment must be carried out by following the "same indications as those defined by clinical trials conducted in France" and in "a limited number of particular situations, which must be the subject of a collegial medical decision at the level of the care unit where the patient is taken care of ”, specifies the Agency.
The US drug agency, the Food and Drug Administration, has also given the green light to test such treatments against SARS-CoV-2 coronavirus.
Newsletter - The essentials of the news
Every morning, the news seen by Le ParisienI'm registering
Your email address is collected by Le Parisien to allow you to receive our news and commercial offers. Find out more
You can ask us all your questions about the coronavirus by filling out our form or directly at the address coronavirus@leparisien.fr. Find all the answers previously published on our dedicated page.