The leading medical journal The Lancet has announced the strengthening of its review process.
This decision comes after having had to withdraw in the spring a highly publicized study on hydroxychloroquine, this treatment touted by Professor Didier Raoult, due to doubts about its reliability.
PODCAST. Coronavirus: has Professor Raoult taught too much?
This fiasco "pushed us to examine
(our)
proofreading processes to [...] further reduce the risks of bad behavior in research and publications", wrote in a text posted Thursday evening the British journal.
Proofreading by a committee of independent experts, the “peer-reviewing”, is supposed to be a guarantee of the quality of scientific publications.
READ ALSO>
How a study on hydroxychloroquine and scooter accidents ended up online for a day
For work using large databases, such as the one on hydroxychloroquine withdrawn in early June, "at least one of the reviewers
(should)
know the details of these data" and be able "to understand and comment on their value and their limitations. in relation to the subject ”of the study.
For larger databases, the Lancet will also use a “
data science
expert
”.
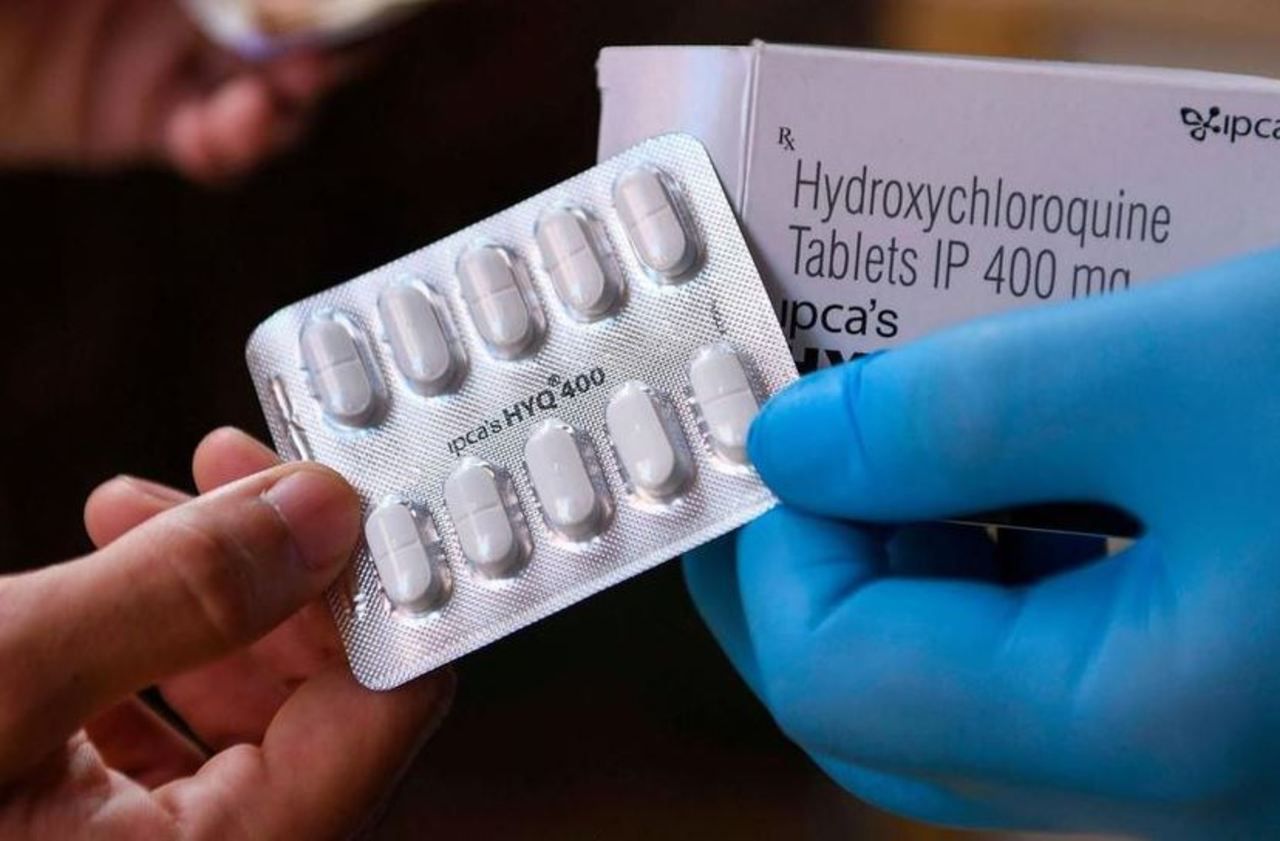
Three of the four authors of the controversial study recanted
At the end of May, the Lancet published a study concluding on the ineffectiveness of hydroxychloroquine against Covid-19 and even on its dangerousness, a work based, according to its authors, on data from nearly 100,000 hospitalized patients around the world .
The World Health Organization had, in the process, halted trials of the drug.
But doubts had quickly arisen around the data collected by the company Surgisphere (whose leader was mentioned as co-author of the study) which had refused to reveal the details.
Three of the four authors had finally "retracted" it and the Lancet had offered its "apologies".
For all studies, the Lancet will also require more written commitments from the authors and that all the authors of the same study engage their responsibility.
For example, “more than one author” must have had “direct” access to the raw data of the study and have “verified” them.
In addition, at least one of those who verified the integrity of the data must be a researcher, unrelated to the commercial entity (a company for example) that would have provided them if necessary.
A document should specify what data will be published, when and how it will be published.
Newsletter - Most of the news
Every morning, the news seen by Le Parisien
I'm registering
Your email address is collected by Le Parisien to enable you to receive our news and commercial offers.
Learn more
VIDEO. Professor Raoult explains himself to the deputies
In another text released on Friday, the Lancet points out that with the pandemic, it has become "particularly complicated" to find reviewers and that, "in some cases, we have received five times more manuscripts than normal ".
To date, the effectiveness of hydroxychloroquine against Covid has not been scientifically demonstrated.