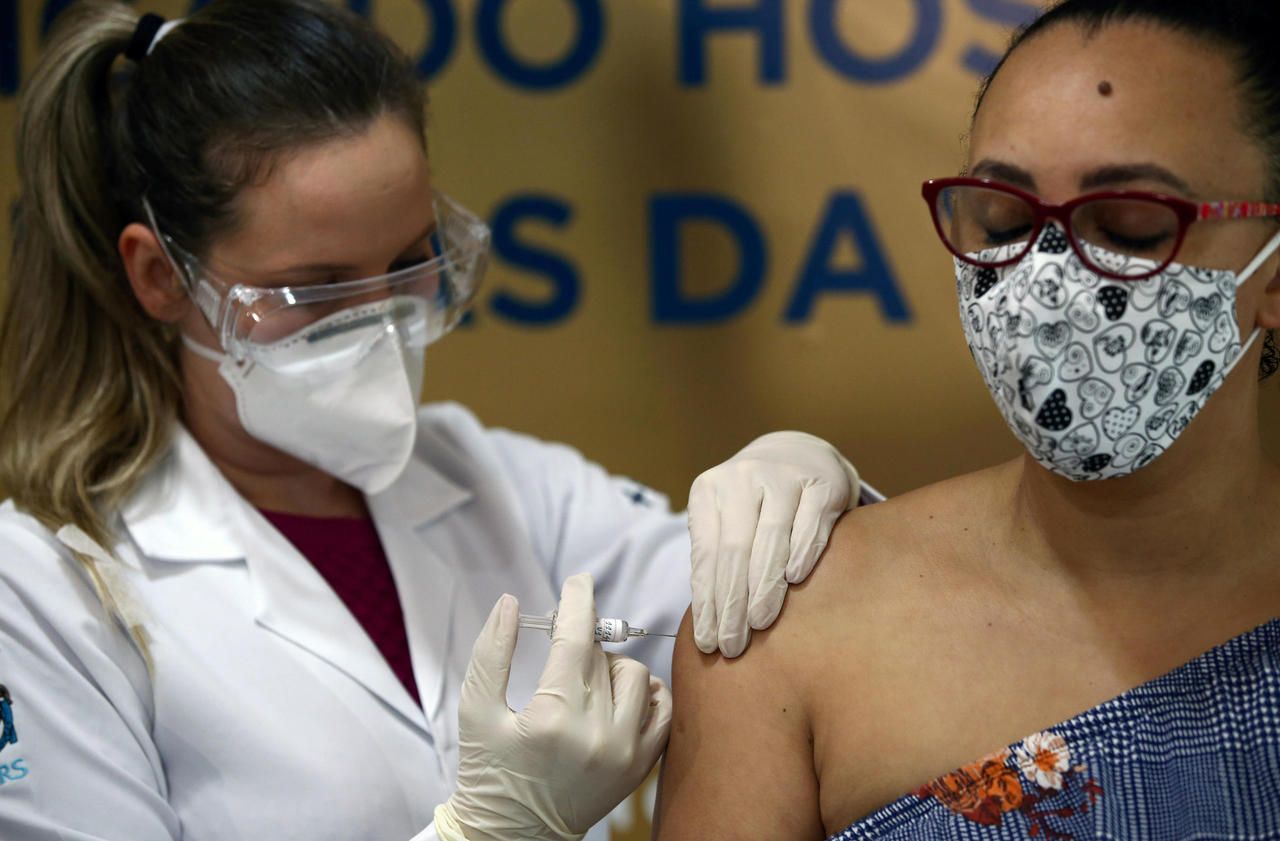
As the race for the vaccine against the Covid-19 continues, the Health Vigilance Agency (Anvisa) "has decided to interrupt the clinical trial of the CoronaVac vaccine after a serious incident" on October 29, she said in a statement.
She did not provide details of what happened, but indicated that these types of incidents could include death, potentially fatal side effects, severe disability, hospitalization and other "clinically significant events." ".
The public body that coordinates vaccine trials in Brazil, the Butantan Institute, said it was "surprised" by this decision.
He will give a press conference on the matter on Tuesday at 2 p.m. (GMT).
This halt for CoronaVac, from the Chinese laboratory Sinovac Biotech, came the same day that the American pharmaceutical giant Pfizer announced that its vaccine against the coronavirus had reached an effectiveness of 90%.
READ ALSO>
A vaccine effective at 90%: four questions on the shattering announcement of Pfizer
For Americans, who have pre-ordered 100 million doses, that means the first vaccinations could begin before the end of the year, provided safety is confirmed, by next week.
Pfizer then planned to submit an application for authorization to the United States Medicines Agency (FDA), which will have to decide whether the vaccine is safe and effective.
Pfizer and Sinovac vaccine candidates are in phase 3 trials, the last stage before they get the green light or not from regulatory authorities.
The two are on trial in Brazil, the second most bereaved country by the pandemic, with more than 162,000 dead.
Political battle around the CoronaVac
The CoronaVac has been the subject of a political battle in Brazil between one of its biggest supporters, the governor of Sao Paulo, Joao Doria, and its main political opponent, President Jair Bolsonaro.
The far-right head of state spoke of Sinovac's vaccine saying it came from "this other country", and instead promoted the one developed by the University of Oxford with the British pharmaceutical company AstraZeneca.
Newsletter - Most of the news
Every morning, the news seen by Le Parisien
I'm registering
Your email address is collected by Le Parisien to enable you to receive our news and commercial offers.
Learn more
Last month, Jair Bolsonaro canceled an agreement to buy 46 million doses of the Chinese vaccine that had been announced by his own Minister of Health.
Referring to "a very discredited China" because "the virus was born there", the president assured that his country was "not going to buy a vaccine that does not interest anyone".
READ ALSO>
Covid-19: the virus escaped from a lab?
Why the thesis is not so far-fetched
On Monday, Joao Doria announced that the first 120,000 doses of CoronaVac would arrive in Sao Paulo on November 20.
The State of Sao Paulo has an agreement with Sinovac to acquire 46 million doses (6 million produced in China, the others in Brazil).
In a press release, the government of this state "regrets having heard of the decision through the press, instead of having been informed directly by Anvisa", and hopes with the Butantan Institute to learn more about " the real reasons for the suspension ”.
"State government officials fear that Jair Bolsonaro is using technical decisions to delay the immunization schedule for political reasons," the Folha de Sao Paulo newspaper reported, citing relatives of Joao Doria.
Another vaccine candidate was suspended for a while: the one developed by the AstraZeneca group with the British University of Oxford, following the onset of a disease in one of the participants.
The trial was suspended worldwide on September 6, but resumed after a few days in the United Kingdom and in the following weeks in South Africa, Brazil, Japan and finally the United States, the various health authorities estimating that the vaccine was safe as the disease was apparently unrelated to the vaccine.