Dr. Noa Bergman explains what causes Alzheimer's, is there a way to prevent the disease, and how to treat someone who is already ill (Walla system!)
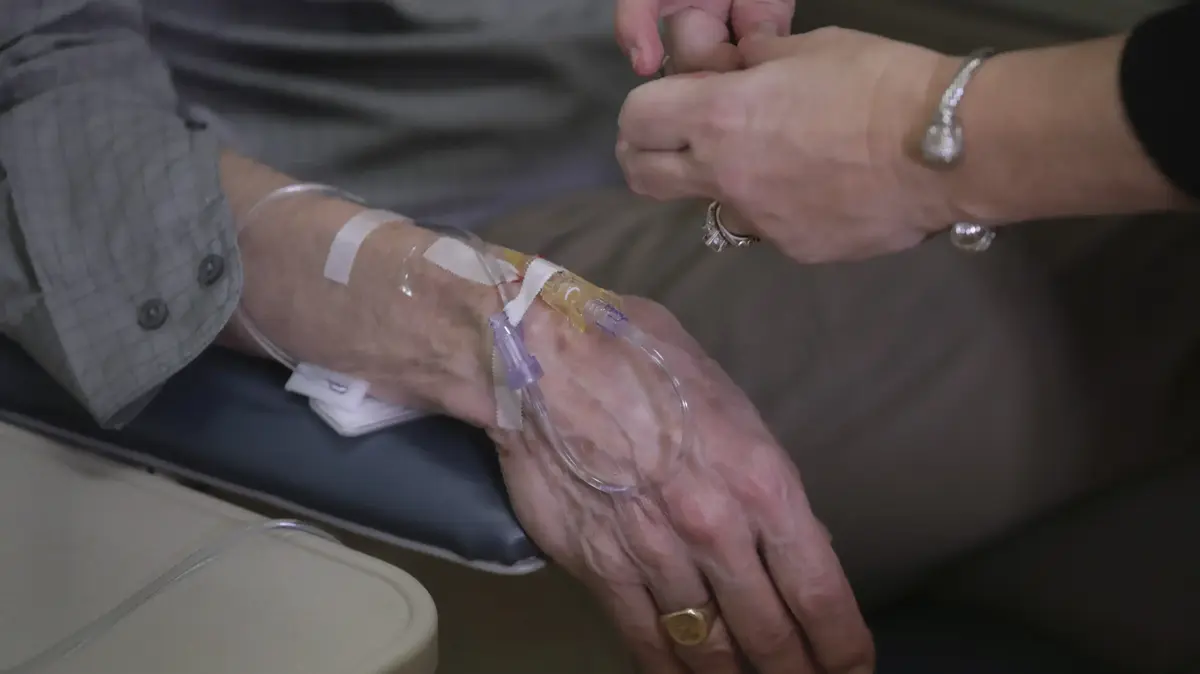
Alzheimer's is a very scary disease, so when it was announced that there is a new antibody that slows cognitive decline in some patients - the enthusiasm was great.
However, a third death related to the drug during its clinical trials increases concerns about its safety.
New medical records revealed that a 79-year-old Florida woman who participated in the antibody trial died in mid-September after experiencing extensive brain swelling and bleeding, as well as seizures.
Neuroscientists who examined the records believe that her death was likely caused by the antibody of the drug, which is called lecanemab and is considered a breakthrough in the field of Alzheimer's care.
"The brain swelling and microbleeds... could be a serious side effect of the study drug, and should be evaluated by trial investigators," Alice van Etten, a neuroscientist and neurologist at Leiden University, told SCIENCE.
The Japanese biotech company Eisai Co, which makes the drug, did not disclose the third woman's death in a celebratory statement last month at the annual Alzheimer's conference in San Francisco.
During the conference, the company revealed data from a phase 3 trial of lecanemab that showed a slowing of the rate of cognitive decline among early Alzheimer's patients, by an average of 27 percent over 18 months.
It is important to say that the newly disclosed death comes amid other reports of serious brain bleeding and swelling in the main clinical trial, and two additional deaths in the other phases of the trial.
Eisai, which attributed the previous deaths and brain injuries to factors unrelated to the drug, declined to comment on the Florida woman's death, citing concerns about patient privacy.
"All serious events, including deaths, are reported to Eisai and considered in our research evaluation," a company spokesperson said in a written statement to Science.
"This information is provided to the FDA (US Food and Drug Administration) and other regulatory authorities, as well as to independent review boards for the study," the spokesperson added.
According to him, the age and medical condition of each trial participant must be taken into account when assessing death.
The Florida woman, however, had no apparent health problems, other than her signs of early Alzheimer's disease, according to her medical records.
More in Walla!
Chris Hemsworth is at increased risk for Alzheimer's.
What exactly does that mean?
To the full article
"The age and medical condition of each participant in the experiment must be taken into account."
Clinical trial of a drug for Alzheimer's (Photo: AP)
Eisai reported 13 deaths in the pivotal clinical trial, in which about 1,800 people participated.
Deaths are expected given the age and health of the study population, and the company says the numbers were similar in the Lecanemab and placebo groups.
But the details of each death have not been published, so in most cases scientists have not been able to independently assess whether the drug caused their death.
Lecanemab is one of several experimental Alzheimer's drugs that target amyloid beta, the protein that accumulates in the brains of people with the disease.
Many believe that beta amyloid is responsible for the death of brain cells that robs people of their memories and eventually kills them, although deposits of the protein are also found in the brains of healthy people.
A few more important things to know about Alzheimer's: For the
first time in 20 years: a new drug for Alzheimer's has been approved.
If you eat these foods, the risk of Alzheimer's increases
Antibodies that damage the amyloid often cause brain swelling and bleeding, a condition known as edematous amyloid-related imaging abnormalities (ARIA) because it is diagnosed using brain imaging.
"We need to change the name ... because these are not just imaging abnormalities, as this case illustrates," said neurologist and neuroscientist Andreas Charidimo of Boston University, who examined the woman's records.
"This is a real clinical syndrome, which can be fatal," he added.
The antibodies that damage the amyloid can be harmful.
Plaque accumulation of beta amyloid in the brain (Photo: ShutterStock)
In the two previous deaths linked to the drug, neurologists said the patients' use of anticoagulants worsened the swelling and bleeding in the brain.
The Florida woman was given a minimal dose of the anticoagulant heparin after she was admitted to the hospital, but several neurologists dismissed it as a contributing factor to her sudden problems and eventual death.
It is unclear whether the woman received an infusion of the antibody or a placebo during the 18-month trial.
But she did receive the drug for 6 weeks in the extension phase - where each participant can choose the treatment.
Before the extension phase began, a brain scan revealed signs of some bleeding, but they were not serious enough to disqualify her from participating in the trial.
The woman's friend described a shocking series of events that began with the first infusion of the antibody as part of the long trial in August.
"She was so tired. She didn't get out of bed for two days except maybe to eat yogurt or go to the bathroom," said the friend.
Two weeks later, after the second infusion, the woman complained of severe headaches, "couldn't complete sentences", and felt increasingly confused in everyday matters, her friend recalls.
On September 14, while at a restaurant, the woman experienced what appeared to be a stroke.
She was rushed to the hospital, where her friend informed the doctors that the woman was taking the experimental drug.
Brain scans showed dozens of areas of bleeding and brain swelling so extensive that the characteristic folds of the cerebral cortex had "fused and crushed" in large parts of her brain.
In light of the lack of other potential causes of brain damage noted in the medical records - there is a very reasonable chance that the cause was the medication.
Hospital records show that the clinical trial investigator, contacted after the woman's hospitalization, suspected ARIA and rushed her to steroid treatment - which doctors tried without significant benefit.
She began to suffer from organ failure and pneumonia, and died 5 days after hospitalization.
health
news
Tags
Alzheimer's
dementia






/cloudfront-eu-central-1.images.arcpublishing.com/prisa/BS6KLR7HWVHDBPE2NL7LDTVNKE.jpg)





