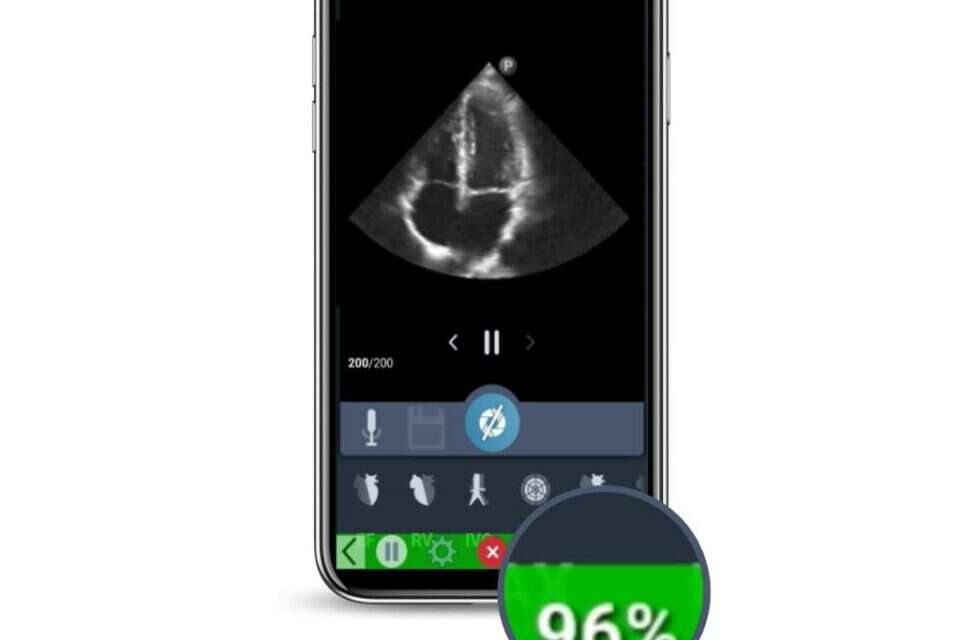
A new Israeli development can help perform higher quality echocardiograms.
The development called "IQS LVivo" uses artificial intelligence to direct the examiner to the exact point where high-quality imaging of the heart will be possible.
The development was approved today (Monday) by the FDA (American Food and Drug Administration)
The software was developed by the company "DIA" (dia analysis imaging) located in Be'er Sheva and headed by 3 female CEOs. The company has now received its ninth approval from the FDA, when the previous approvals concerned the developments created by the company in the field of artificial intelligence as tools for deciphering chest and abdominal ultrasounds.
The current software helps the ultrasound operators perform high-quality heart imaging, while scanning, to assist in the process of clinical evaluations of the heart in real time, which will provide an answer to the challenges that arise in the examination due to the constant movement of the heart and its deep location in the chest.
Since the results of the test also depend on the different experience of the testers, the new development will allow even those who are less skilled to perform the test in a high-quality way.
We gave the ninth approval to the Israeli startup, photo: E.P
Hila Goldman-Etzlan, CEO and founder of DIA, said about the approval: "Our complete software in the field of cardiology today helps ultrasound users overcome two major challenges in the field - high quality image scanning and accurate analysis of the images.
Receiving FDA approval for this software is a big step towards the company's vision to make both the scanning process and the decoding process of the ultrasound images smarter and more accessible, in the field of cardiology and in other fields."
The FDA approved IQS LVivo after a clinical study conducted at Soroka Hospital that proved its safety and effectiveness.
The results of the study showed a high correlation between the scan quality score obtained from the software and the ability of an expert echocardiologist to give a clinical assessment from the same scan.
91% of the images saved by emergency medicine physicians who used the software were found to be good quality images that could be evaluated clinically.
Dr. Lior Fox, a senior intensive care physician at the Soroka Medical Center in Israel and principal investigator of the study added: "For accurate diagnosis and treatment of the heart, high-quality cardiac ultrasound imaging is necessary both in an emergency environment and in other environments.
The software will help us to scan cardiac ultrasound images in a high-quality, fast and clear manner to assist in our clinical decision-making process in real time."
were we wrong
We will fix it!
If you found an error in the article, we would appreciate it if you shared it with us