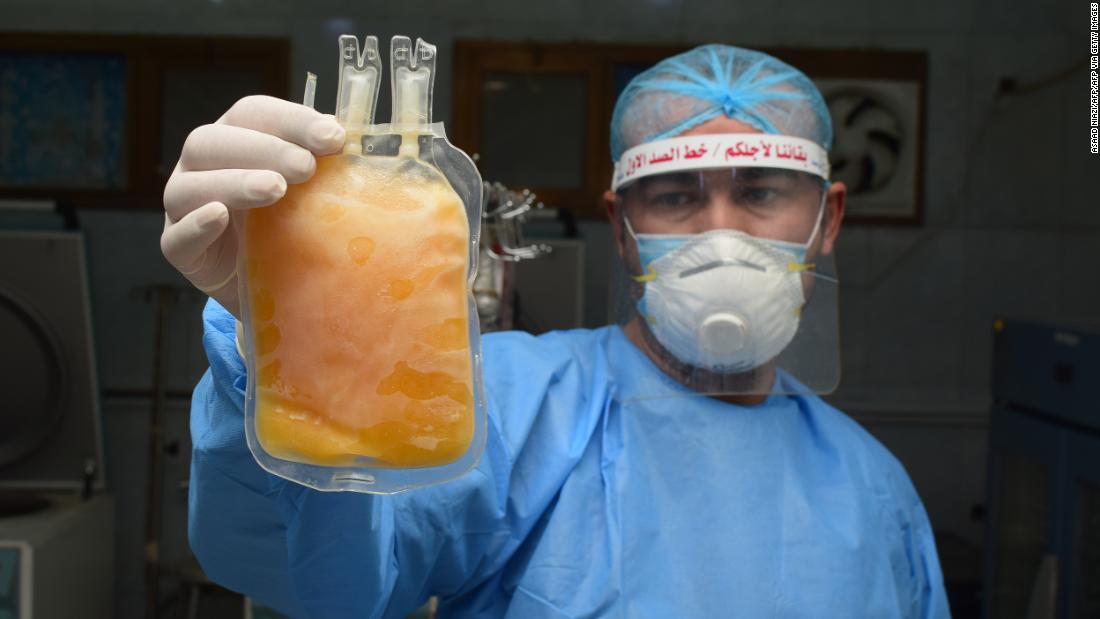
US stops use of plasma from recovered patients 0:30
(CNN) - A U.S. Food and Drug Administration (FDA) emergency use authorization for blood plasma to treat COVID-19 has been suspended, but could still be issued in a near future, Dr. H. Clifford Lane, deputy director of the National Institute of Allergy and Infectious Diseases, told The New York Times.
The suspension came after a group of federal health officials, including the director of the National Institutes of Health, Dr. Francis Collins, the director of the NIAID, Dr. Anthony Fauci and Lane, intervened to argue that the data Emerging data on treatment were too weak, the Times reported Wednesday, citing two senior administration sources.
"All three of us are quite aligned with the importance of robust data through randomized control trials, and that a pandemic does not change that," Lane told the Times.
Dr. Anand Shah, FDA's deputy commissioner for medical and scientific affairs, noted in an emailed statement to CNN on Wednesday that while the FDA and NIH collaborate "in several areas" of the government's response to COVID -19, NIH does not participate in regulatory decisions of the FDA.
“In general, NIH are not involved in the decision-making process at FDA and do not have all of the confidential data that the FDA uses to make these regulatory decisions. We take seriously our mandate to follow data and science in reviewing medical products to prevent or treat COVID-19 according to the legal and regulatory standards set by the agency, ”Shah said in the statement.
"As per policy, we cannot comment on whether we will take any action regarding the emergency use authorization for convalescent plasma and will make a decision at an appropriate time," he said. "Notably, the safe use of convalescent plasma remains available to patients through multiple avenues, including clinical trials, an expanded national access protocol, or through a single-patient emergency investigational new drug application." .
The treatment is created from the blood of people who have recovered from Covid-19 and has been successful in two other deadly coronaviruses, MERS and SARS. It has also been used to treat the flu.
FDA emergency use authorization does not require the same level of evidence as full FDA approval. The agency previously granted an emergency use authorization to hydroxychloroquine and chloroquine for the treatment of COVID-19, then rescinded it in June after antimalarials were found to be ineffective against the coronavirus.
In late March, the FDA created a pathway for scientists to test convalescent plasma with patients and study its impact. Doctors have used the treatment ever since. USCovidPlasma.org reports that nearly 67,000 people have been infused with the treatment and nearly 14,000 physicians use it as part of a Mayo Clinic program, but it's not yet clear if it works. Several studies are underway.
President Donald Trump and US healthcare leaders, including Fauci and other members of the White House coronavirus task force, and even celebrities like Dwayne "The Rock" Johnson, have encouraged people to survived the covid-19 to donate plasma. Two weeks ago, on a Red Cross tour, Trump implored people to donate plasma, saying, “We have a lot of people who would heal, get better. As soon as I can, please.
The United States government has invested money in the experimental treatment. It awarded Plasma Technologies LLC a defense contract worth $ 750,000 to develop expanded covid-19 plasma technologies, according to an announcement on the Department of Defense website on Monday. It also stores 200,000 units of convalescent plasma, and the Defense Department said it wants to collect more by September.
The first results of some small studies look promising.
A very small study published in JAMA in March showed that four out of five patients who received convalescent plasma improved. A second, very small study of 10 Chinese patients published in May in the Proceedings of the National Academy of Sciences found improvement in patients treated with convalescent plasma, without safety concerns. Another May study of 25 patients in Houston showed that patients improved without safety concerns. In July, a larger study of 5,000 hospitalized patients showed that the treatment was safe.
Not all trials have reached the same conclusions. A small study by Chinese researchers published in June did not show an improvement in the death rate among six critically ill patients, but the researchers did not completely rule out convalescent plasma. Instead, they suggested that it might work if patients were treated earlier.
Another randomized trial of 103 patients in China found no statistically significant improvement in patients, but it is difficult to draw conclusions from that trial because it had to stop early when the flow of patients decreased and there were not enough to test the therapy. Some other studies in the US have also had trouble recruiting enough patients.
There is a larger study published in August involving 35,000 patients that has not yet been peer-reviewed. It found that giving people early plasma treatment appeared to further reduce deaths. People who received the transfusions within three days of diagnosis had a seven-day death rate of 8.7%, while patients treated four or more days after the course of the disease had a death rate of 11.9%.
But there was no placebo group in the study. Without that comparison, it is still difficult to know if the treatment made a difference.
"Convalescent plasma has yet to be shown to work with the trials we want to see, these randomized control trials," said Dr. Ian Lipkin, director of the Center for Infection and Immunity at the Mailman School of Public Health at the University of Washington. Columbia, in an interview Monday. "While there are plenty of reasons for optimism there too, it has not been proven and until it is done there will always be healthy skepticism in the community, despite all the background information suggesting that it is likely to work."
covid-19 Plasma