World theory
Written by: Ou Jingluo
2020-09-06 17:31
Last update date: 2020-09-06 17:57
The Hong Kong government announced on September 4 that it had submitted a letter of intent to the global access mechanism for new coronavirus pneumonia (COVID-19) vaccines (COVAX) as part of Hong Kong's vaccine procurement strategy.
COVAX is a project led by the World Health Organization (WHO) and international vaccine-related organizations to promote the development, production and distribution of new crown vaccines.
There are currently 9 vaccines included in COVAX.
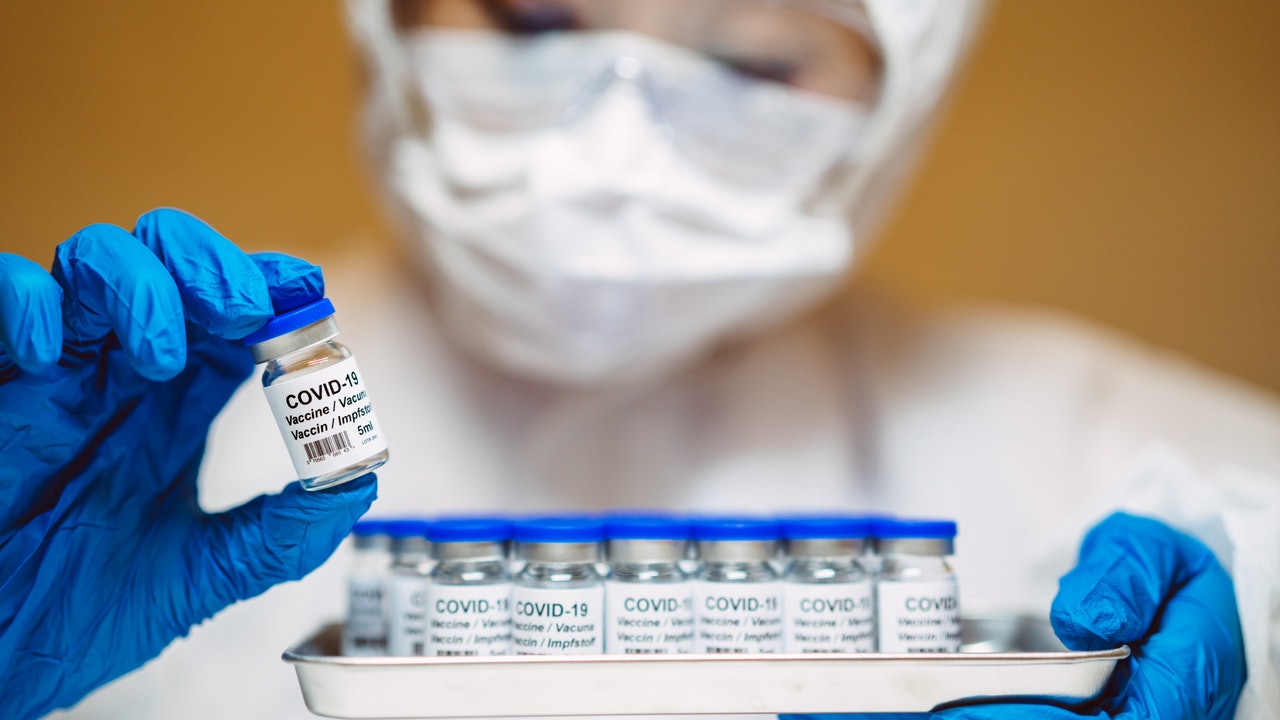
The picture shows the new crown vaccine developed by Sinopharm Group China Biotechnology on September 5.
The vaccine has now entered the third phase of human testing, but it is not in the COVAX plan.
(Getty)
So far, more than 30 candidate vaccines are currently undergoing human testing, and at least 8 have entered the third phase of testing, but not all vaccines have participated in the COVAX program.
Including Russia’s recently published papers proving that the vaccine is initially effective, the "Sputnik-V" vaccine, as well as China’s Cansino Biology, Kexing Biology and Sinopharm’s vaccines, are currently not included in the COVAX list.
According to WHO, the 9 vaccines currently in COVAX are all funded by the Epidemiological Prevention Innovation Alliance (CEPI).
Among the 9 vaccines, only one vaccine comes from China, one from Hong Kong, one from Sino-US cooperation, and the rest from Europe, America and other countries.
Only two vaccines entered the third phase of the experiment.
The picture shows the art picture of the vaccine developed by Moderna, USA in June.
The vaccine has now entered the third phase of human testing.
(Getty)
Vaccine: mRNA-1273
Main development: Moderna
Research: Phase 3
The vaccine developed by Moderna Pharmaceuticals in the United States is currently one of the leading vaccines in the COVAX program.
The first and second phases of the experiment were carried out in March and May respectively, and both achieved satisfactory results.
The third phase of the experiment was launched at the end of July, and the target was 30,000 American nationals.
Vaccine: ChAdOx1 nCoV-19
Main development: Oxford University
Research: Phase 3
The University of Oxford in the United Kingdom focuses on developing vaccines. It is one of the leading vaccines in the COVAX program along with mRNA-1273. The third phase has been launched in the United States in August.
The British government attaches great importance to vaccines and is ready to put it into production. If it is formally approved, a certain vaccine can be provided as soon as September for medical care and frontline personnel to administer it.
Vaccine: CVnCoV
Main development: CureVac
Research: Phase 2
The vaccine developed by the German CureVac Pharmaceutical Factory has now entered the second phase of experimentation.
The company went public on August 14 in the United States, and it rose 2.5 times on the first day of listing, becoming the representative of the European new crown vaccine.
CureVac has pledged to invest more resources to accelerate development.
↓↓↓If
you want to know the lives of people in other countries during the epidemic, please click to enlarge:
[New Coronary Pneumonia] WHO: Never "endorse" an unproven safe and effective vaccine
[Russian vaccine] Defense Minister Shoigu vaccinated the country's first registered vaccine
【Coronavirus disease.
Multiple pictures] Several domestic vaccines appeared in China International Trade Fair
【New Coronary Pneumonia】WHO: Vaccine nationalism is not good for fighting the epidemic
Vaccine: INO-4800
Main development: Inovio Pharmaceuticals, Beijing Aidiweixin Biotechnology
Research: Phase 2
The INO-4800 vaccine was jointly developed by China and the United States. The first phase of the trial started in the United States in April, and the second phase was tested in South Korea in June.
The first phase of the experimental study indicated that 94% of the experimenters had obtained an immune response and are now waiting for the second phase of the experimental results.
Vaccine: NVX-CoV2373
Main development: Novavax
Research: Phase 2
The vaccine developed by Novavax in the United States entered the second phase of trials in August.
The second phase of the test was conducted in South Africa, with a total of 2,904 people participating, and the test results are currently awaited.
The company stated that it is conducting experiments in Africa because Africa has entered the peak of respiratory diseases in the winter, and it hopes to prove whether the vaccine is effective in African residents.
Vaccine: TMV-083
Main development: Institut Pasteur, Merck Pharmaceuticals, Themis
Research: Phase One
The TMV-083 vaccine is a vaccine jointly developed by France, the United States and Austria.
The first phase of the vaccine was tested in France and Belgium in August, with 90 people participating, and the results are currently awaited.
↓↓↓If
you want to know the lives of people in other countries during the epidemic, please click to enlarge:
New crown pneumonia | The government announced that it will participate in the COVAX mechanism to obtain vaccines and then purchase vaccines from pharmaceutical companies
Wang Yi: The new crown vaccine should not be monopolized by any country, nor should it be exclusively enjoyed by rich countries
[New Coronary Pneumonia] WHO expects widespread vaccination in mid-2021, Tan Desai to promote fair distribution
[New Coronary Pneumonia] The US vaccine was approved in October?
Fauci: unlikely but not impossible
Vaccine: SCB-2019
Main development: Clover Biopharmaceutical
Research: Phase One
China Clover Biopharmaceuticals developed a vaccine and entered the first phase of human trials in Australia in June.
The company expects to enter the second phase of the experiment in August, but there is no further news for the time being.
Vaccine: Molecular clamp stabilized Spike protein
Main development: University of Queensland
Research: Phase One
The name of the vaccine is also called UQ-1-SARS-CoV-2-Sclamp, which is mainly developed by the University of Queensland in Australia.
The first phase of the vaccine was tested in humans in Australia in July.
The University of Queensland issued an announcement in August, calling on more seniors to join and participate in the expansion experiment. It is believed that the vaccine will enter the second phase of the experiment.
Vaccine: RBD replicating influenza vector
Main development: The University of Hong Kong
Research: No human testing
The vaccine was developed by the State Key Laboratory of Emerging Infectious Diseases, Department of Microbiology, School of Medicine, University of Hong Kong. In March, the team received a seed fund of USD 620,000 from CEPI to fund pre-clinical testing of the vaccine.
The vaccine is still in the development stage and has not yet entered human testing.
New crown vaccine vaccine new crown pneumonia global epidemic news classroom