60 premature babies transferred in Brazil due to lack of oxygen 0:45
Sao Paulo, Brazil (CNN) -
Amid a devastating coronavirus resurgence in parts of Brazil, federal health officials in the South American country finally voted to authorize two vaccines for emergency use.
On Sunday, the Brazilian regulatory agency Anvisa approved both the Oxford / AstraZeneca vaccine and the Coronavac vaccine.
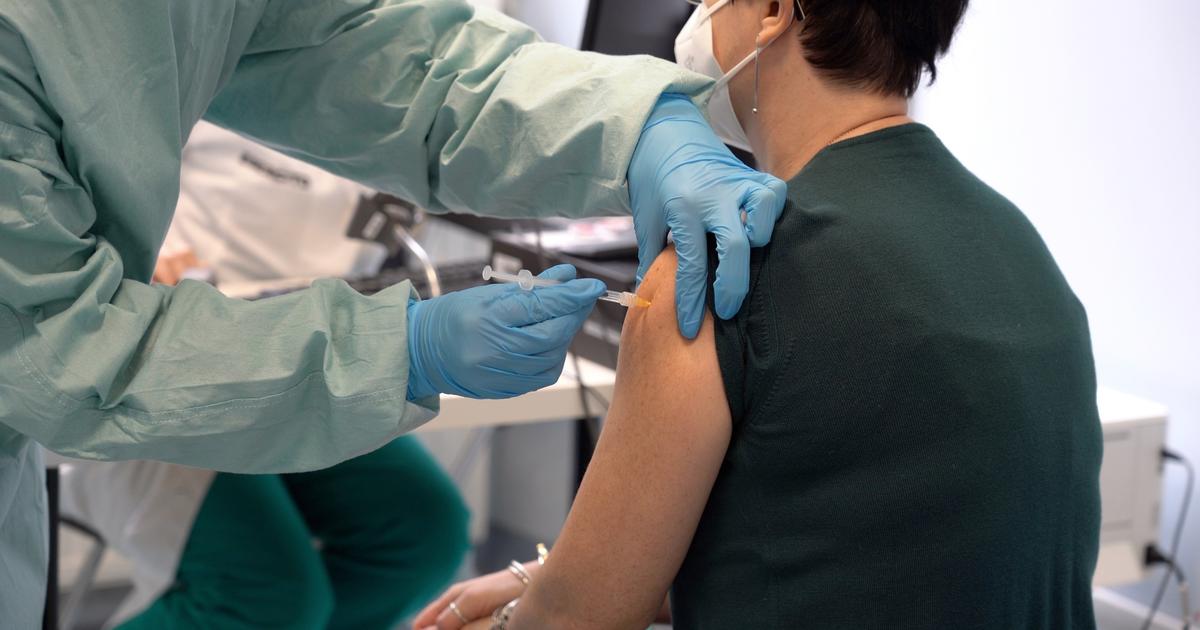
Minutes later, Monica Calazans, a black nurse from downtown São Paulo, became the first Brazilian to be vaccinated.
Calazans, who is at high risk of complications from Covid-19 and works in an intensive care unit (ICU) that has been at 90% capacity or more since April, broke down in tears before receiving the Coronavac injection.
The United Kingdom prohibits entry of travelers from several Latin American countries due to the Brazilian variant of covid-19
"They don't know what this means to me," he told São Paulo state governor João Doria.
Nurse Monica Calazans receives the first injection of the Coronavac vaccine in Brazil at the Hospital das Clínicas of the University of Sao Paulo (USP), on January 17, 2021.
Brazil, the most affected country in the region
Brazil is the country most affected by covid-19 in Latin America.
It has registered more than 8 million cases and more than 200,000 deaths from coronavirus.
While several of its neighbors have already approved the use of vaccines, Brazil appears to be lagging behind despite its recognized record of public health and vaccination.
The Coronavac vaccine, developed by the Chinese company Sinovac, has now been licensed for the use of 6 million imported doses.
It has a history in the state of Sao Paulo, where the Butantan Institute conducted phase 3 clinical trials of the vaccine.
Butantan will also produce future doses.
However, Coronavac has shown a low average efficacy rate of 50.4%, just above the 50% minimum set by the World Health Organization.
The number, which falls well below the previously announced 78%, has raised questions about the veracity of the data and fueled skepticism about the apparent lack of transparency regarding Chinese vaccines.
advertising
Sinovac's Chinese vaccine has low effectiveness, according to Brazilian health authorities
Anvisa's technical report that gave the green light to Coronavac underlines that the agency had also taken into account the urgency due to the vertiginous increase in cases of covid-19 in Brazil and "the absence of therapeutic alternatives."
It also recommends that the vaccine be monitored.
It indicates that the Butantan Institute had not provided important data from its Phase 3 study, such as the duration of protection provided by the vaccine and its effect on older adults, people with comorbidities, and other patient groups.
Doria has committed to making the vaccines developed in the state available to the Brazilian federal Ministry of Health for national distribution.
'Day V'
"Today is Day V. It is the day of the vaccine, it is the day of truth, it is the day of victory, it is the day of life," he said at a press conference after the vaccines were approved.
It was a sharp blow to the reluctance of the Minister of Health, Eduardo Pazuello, to commit to a national vaccination start date, which he previously said would begin "on D-Day and H."
Brazil would produce the Sputnik V vaccine to sell it to Argentina and Bolivia
The Oxford / AstraZeneca vaccine, which showed an average efficacy of 70.4% in preliminary Phase 3 trials, has also been approved for the use of 2 million doses, to be imported from the Serum Institute of India by the Foundation Oswaldo Cruz from Brazil (Fiocruz).
Fiocruz signed an agreement to buy and produce the vaccine with pharmaceutical giant AstraZeneca in June.
After successive delays, the Brazilian government signed a contract for 256 million doses in October and announced that it would receive the first in December.
After another series of delays, Fiocruz expects to receive the first shipment in late January.
CNN's Rodrigo Pedroso reported from São Paulo and Caitlin Hu reported from New York.
Brazilvaccine against covid-19