Note to readers: EL PAÍS offers openly all the content of the Future Planet section for its daily and global contribution to the 2030 Agenda. If you want to support our journalism,
subscribe here.
On this day, March 24, 1882, the German physician Robert Koch presented a revolutionary finding at the Berlin Physiological Society: the
mycobacterium tuberculosis
bacillus
, the cause of the infectious disease of the same name that in the 19th century was responsible for a out of every four deaths.
Some time later, in July 1921, a French boy became the first person to receive a dose of an experimental vaccine called Bacilo Calmette-Guérin or BCG in honor of its creators, the microbiologist León Charles Albert Calmette and the veterinarian Camille Guérin.
Starting from inoculation in patients with the live and weakened bovine tuberculosis microbe, it was shown that it could generate antibodies in humans, but with limitations: it only protects children in the most serious forms, not adults, and it does not work against the disease. most common form of the disease, pulmonary.
Despite the fact that the investigation to find a better substitute has never stopped, a hundred years later a replacement has not yet been found and tuberculosis has become the infection that causes the most deaths in the world -except for 2020 due to the covid- 19–, with 1.4 million deaths in the last year.
In the last two centuries it has caused more than a billion victims.
More information
Vaccinating the future
Economic losses from tuberculosis are calculated for the first time
"The investment that Spain has made against pandemics can be lost"
Although the comparisons are odious, it is inevitable to make them after seeing the mobilization that has been carried out to find a remedy against covid-19, with at least 82 developments in the clinical phase in just one year.
Meanwhile, for tuberculosis there are between 10 and 15 after a century of research.
“After a long time we are starting to have options that, if they pass the final stages of clinical trials, should be available in five or six years.
The question now is, with the experience we have obtained with covid-19, to see what it takes for tuberculosis to continue on the same path ”, asks Cesar Ugarte-Gil, epidemiologist at the University's Institute of Tropical Medicine Peruvian Cayetano Heredia.
Although it may seem little, this is the moment in history in which there is more research in progress thanks to the progressive increase in funding and international visibility but still insufficient to end this endemic disease, warns Alberto García Basteiro, epidemiologist and researcher at the Barcelona Institute for Global Health (IS Global).
The World Health Organization has set its eradication goal for 2050, although current trends suggest that this goal will not be achieved.
Meanwhile, the search for a new vaccine is progressing slowly.
The two winning bets
Dr. Ugarte assures that, if he had to bet, he would do so on two promising vaccines, and one of them is Spanish.
It is the MTBVAC, the only one that uses the live and attenuated bacillus of
Mycobacterium tuberculosis
and which has been designed following the method of the creator of these drugs, Louis Pasteur, in the 19th century: it is a bacterium to which it is they have removed the genes that make it dangerous.
This has been designed by the genetic research group of Dr. Carlos Martín Montañés, from the University of Zaragoza, and Brigitte Gicquel from the Institut Pasteur in Paris, and developed by the Biofabri laboratories.
The research began 20 years ago, a first safety test was done in adults in Switzerland and in 2015 it was tested on a larger scale in South Africa, one of the most endemic countries, showing that it is just as safe as BCG.
Currently, the research team has just completed phase IIA of its clinical trial, carried out on 99 babies in the African country;
in September it ended in another 144 adults.
In scientific jargon, phase I is to evaluate safety, and phase II examines immunogenicity, that is, the ability of an antigen to activate the immune system and induce an immune response.
Although there are still a few months ahead to obtain definitive results, these are expected to be optimistic in the opinion of Esteban Rodríguez, CEO of Biofabri.
“The impression is that [the vaccine] is extremely safe.
We have seen that in both adults and children, even with doses of 10 high [that is, injecting one hundred thousand germs at one time into a person] has been shown to be safe ”, celebrates Rodríguez.
A study in macados has shown for the first time that the Spanish vaccine protects against respiratory infection, the most common, and that it does so better than BCG
But there is another equally important finding: "We have also seen that immunogenicity parameters are repeated in all individuals," he adds.
These results should also be read taking into account another study of MTBVAC in macaques, which have an immunity similar to that of humans, and which was published in January 2021 in the scientific journal
NPJ-Vaccines.
This has shown, for the first time, that Spanish protects against respiratory infection, the most common, and that it does it better than BCG.
“What has been seen is that the parameters of the macaques vaccinated with MTBVAC are equivalent to those we have observed in children and adults, with which this is indicating that, for the first time, correlations of protection in tuberculosis disease, this is very important ”, celebrates the CEO of Biofabri.
"The study of macaques for us is white and in a bottle, and that is why we are very excited that the efficacy study is going to work," says Dr. Carlos Martín, referring to the next phase of this research.
“Now we have to define the correct dose for the vaccine;
We hope that within six months, when we have the data, it can be determined ”.
The results of phase III will take about five or six years to conclude: "We have to think between one or two years until the disease develops and then only 5 to 10% of individuals will develop it," he says. the microbiologist.
The M72 vaccine has shown 54% protection against active pulmonary tuberculosis disease
The Spanish-made vaccine is not the only promising vaccine.
On the campus there is another one that a year ago announced that it had passed several phases of clinical development in humans, called M72, from GSK laboratories and supported by the Gates Foundation.
The latest results, published in December 2019, revealed that in the 3,289 infected adults in South Africa, Kenya and Zambia, 54% protection was protected against active pulmonary tuberculosis.
Although these are encouraging results, García Basteiro warns: “The study says that all the efficacy was found in the cases in South Africa, because in the other two countries it demonstrated protection, with which we must be cautious and wait for the result of the next phases of clinical development, III ”.
Dr. César Ugarte agrees: "We have to see how it behaves in a large clinical trial because, although 54% protection is enough for what is requested, obviously you want a little more."
What is missing to achieve a new vaccine?
The slowness in the search for a better vaccine is explained by factors such as lack of funds, says García Basteiro.
"If we had enough money, there would be many more candidates and many more trials that could eventually identify one that was really very promising."
In fact, on World Tuberculosis Day, which is celebrated this March 24, the WHO has once again emphasized the need to invest at least 13,000 million dollars each year to end the disease, as well as allocate at least another 2 billion in research for better science, better tools and better results.
Míriam Alía, an expert in vaccination at Doctors Without Borders, agrees with García Basteiro on the lack of funding and asks for something more: better collaboration mechanisms with high-prevalence countries, because vaccines must be tested where the disease is most frequent.
“They also need to be useful for those who get sick the most and suffer the highest mortality, which are patients with HIV and other chronic diseases, and that they be easy to apply, that protect against all possible forms of tuberculosis, especially severe ones.
And, if it is possible - it ends - that they are thermostable and cheap vaccines to facilitate transport them to the destination countries ”.
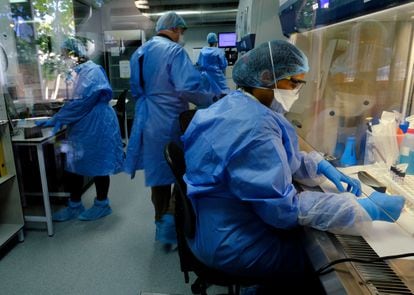
Scientists study the BCG vaccine against tuberculosis at Task laboratories in Cape Town, South Africa.MIKE HUTCHINGS / Reuters
The second factor that complicates finding a better immunization is the nature of the bacillus: "At a technical level it is difficult to test anti-tuberculosis drugs in clinical trials because you have to wait for someone to develop the disease, when these episodes are not so frequent" indicates García Basteiro.
One of the factors that works against the researchers is that tuberculosis is a disease that takes a long time to generate symptoms, and that, in addition, only about 5% of those who have the bacillus in their body end up developing the infection.
“You need very expensive studies because you have to follow a population in which the incidence can be, in the best of cases, 1%.
Imagine the thousands of participants you have to include to be able to compare a vaccine group with a placebo group ”, he warns.
Old, but not bad
Meanwhile, BCG is still being used and it is mainly because there is no other.
In fact, it is mandatory to inject it to newborns in all countries where the incidence is high.
"It is not only available in Australia, Europe except Portugal and the United States," says Dr. Martín.
However, there are other valuable reasons: "It has been seen that it has nonspecific effects on other diseases, such as leprosy, and there have also been many studies that show that it strengthens the immune system against infections, especially respiratory infections", adds Alía, of MSF.
Even when covid-19 appeared, there were some studies to see if BCG could protect, since a correlation was observed between the countries that inoculate it and lower rates of infection of the new coronavirus, although it was later proven that it was not relevant.
Carlos Martín goes further: "BCG is used in bladder cancer treatments, and last year we demonstrated with the Carlos III Health Institute that it protected against pneumococcus in mice," he recalls.
“We have also published in the
EBiomedicine
magazine
that improves the immunity of the DTP vaccine against diphtheria, tetanus and pertussis ”.
It refers to an investigation that has proven that in countries where both immunizations have been combined, there has been up to ten times less incidence of pertussis than in those where anti-tuberculosis is not administered.
FUTURE PLANET can follow on
,
and
, and subscribe
here
to our 'newsletter'
.