Most effective vaccines against covid-19 ... so far (Video from February 2021) 0:46
(CNN) -
At the headquarters of the biotech company Novavax, scientists are developing what they hope will soon be another COVID-19 vaccine for the United States and the world.
Data from the company's large-scale phase 3 clinical trial of the vaccine in the United States and Mexico are expected this month, but the timeline depends on how quickly data on the prevalence of the disease is accumulated in the areas of trial.
The company's COVID-19 vaccine has been in the works for a year, Dr. Gregory Glenn, president of research and development at Novavax, told CNN.
The work began even before the world realized it was facing a pandemic.
In January of last year, Glenn and his colleagues were taking a close look at an unusual cluster of pneumonia reported in China.
They wondered if that outbreak could be due to another version or recurrence of the coronavirus that causes severe acute respiratory syndrome, or SARS.
"It occurred to us that this could be 'SARS 2'," Glenn said.
“The sequence was published on the Internet, the genetic sequence of the virus, and we could see that it was a coronavirus.
Then we went into action.
Novavax says its covid-19 vaccine is 89% effective in a UK trial, but less effective in South Africa
Pfizer confirms the protection time of its vaccine 1:01
How does the Novavax vaccine work?
Using moth cells
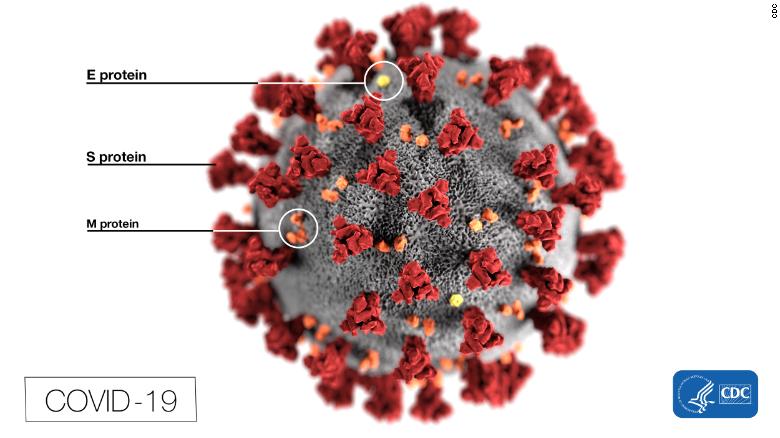
When the sequence of the virus was published online, scientists around the world quickly identified the SARS-CoV-2 virus, which causes covid-19, as a coronavirus because it has what are called "spike proteins" on its surface.
These spikes form large bumps that give coronaviruses the appearance of wearing crowns, which is why they are called 'coronaviruses'.
advertising
These button-shaped structures are what the virus uses to connect with human cells and cause infection.
Getting the immune system to recognize and "remember" those spike proteins is key to how a vaccine helps protect against COVID-19.
However, the way the various coronavirus vaccines do it can vary.
This illustration, created by the CDC, reveals the spikes that adorn the outer surface of the virus.
Novavax's coronavirus vaccine, called NVX-CoV2373, differs from the three vaccines already in the United States.
The vaccine is based on what is called recombinant nanoparticle technology and Novavax's adjuvant, called Matrix-M, to stimulate an immune response and stimulate high levels of neutralizing antibodies.
When the genetic sequence of the coronavirus was published, Novavax scientists identified the spike protein gene and created a modified version of that gene.
The researchers cloned the genes into a baculovirus that infects insects.
They then infected the moth cells, specifically the cells of the fall armyworm insect, with that virus, leading them to produce the coronavirus spike protein.
These virus-like nanoparticles were harvested to make the Novavax vaccine.
“The general idea of the vaccine is to show the immune system something that looks, tastes and acts like a virus, except that it doesn't make you sick.
So we made the spike protein.
We put it in a particle, basically, like a soap bubble, and it's the size of the virus, "said Glenn.
Covid-19 vaccines: what is the most effective vaccine?
This is how Johnson & Johnson's viral vector vaccine works 0:51
«It is not contagious.
We never touch the coronavirus itself, "he added.
'Then that is given to people, and they produce an immune response that is very focused on just the spike, and I'd say the hallmark of our vaccine is that it gives a very strong immune response with very few side effects, and the dose is very small and the vaccine can be stored at normal refrigerated temperatures. '
That's different from covid-19 vaccines made with messenger RNA (mRNA) carried in fatty particles called lipids.
They are more fragile and must be kept frozen.
The two mRNA vaccines that were licensed in the United States last year, Pfizer / BioNTech and Moderna, use genetic material to stimulate an immune response.
When the vaccine is injected into a person's arm, that genetic material is taken up by muscle cells in the arm, which then follow genetic instructions to produce small pieces of the spike protein.
These small proteins stimulate an immune response, generating antibodies and immune cells that remember what they are like and that will be ready to respond quickly in the event of a new attack.
Johnson & Johnson's single-shot vaccine, which was licensed in February, uses a weakened common cold virus as a vector to deliver genetic instructions to cells in the arm, making the pieces that look like part of the spike protein. coronavirus.
Don't panic if you have these side effects from the covid-19 vaccine - they can be a good sign
"We were a small company"
Novavax expects to apply for an emergency use authorization for its COVID-19 vaccine sometime in the second quarter of this year.
But the company is already manufacturing the vaccine at 10 sites in eight countries, with two sites in the United States: North Carolina and Texas.
"They're all at different stages," Glenn said.
“Some are older, but all are up and running and working on the vaccine.
Therefore, we hope to have a very large capacity.
Novavax's coronavirus vaccine was not among the first licensed in the United States because the company had to train the staff necessary to develop such a new vaccine, Glenn said.
"We were a small company," he said, compared to some of the larger vaccine manufacturers, such as Pfizer and Johnson & Johnson.
"We had to recruit people and our funding was somewhat low," he said.
"In addition to all the challenges of developing the vaccine, which is really complicated, we had the challenge of building a company."
But Novavax "has gotten to a really good point now," Glenn said.
Novavax vaccine has high efficacy
Phase 3 of Novavax's covid-19 vaccine trial in the United States and Mexico has enrolled 30,000 volunteers at more than 100 locations.
A final analysis of data from another large phase 3 trial in the UK showed that the Novavax vaccine has an overall efficacy of 89.7% in preventing mild, moderate and severe disease.
That finding was announced last month.
The Novavax UK trial, with more than 15,000 participants aged 18 to 84 years, found the vaccine to be 96.4% effective against mild, moderate and severe disease caused by the parent and coronavirus strain. 86.3% against the B.1,1.7 variant first identified in the UK, the company said.
In their vaccine trials, Moderna saw 94.5% effective, Pfizer 95% effective, and Johnson & Johnson 66% effective.
However, the Novavax and Johnson & Johnson vaccines were studied later in the pandemic after researchers identified the emergence of the coronavirus variants, compared to the Moderna and Pfizer vaccines that were previously studied.
Among the vaccines, the trials also differed where they were studied, which may help explain differences in efficacy.
The Novavax UK trial also showed that 14 days after the first dose, the vaccine's efficacy was 83.4% in the trial, Novavax said.
Novavax expects to see similar test results in the United States, especially as the B.1,1.7 variant continues to circulate, Glenn said.
'In the UK the strain called B.1,1.7 became predominant and that strain appears to be more severe in terms of causing more deaths than the original strain.
That strain is also taking over in the United States.
Some people expect that by April all or most of the virus will be this new B.1,1.7 variant, "said Glenn.
"We are confirming our high efficacy against that strain by 86%," said Glenn.
That's really encouraging for our data from the United States.
It suggests that our test in the United States, even if the virus evolves, will look great. "
The efficacy of the Novavax vaccine against the South African variant
The company also noted that in an analysis of a smaller-scale Phase 2b trial in South Africa, where variant B was first identified.1,351.
The vaccine was 55.4% effective in HIV negative people.
In both trials in the UK and South Africa, the vaccine offered 100% protection against severe COVID-19 that resulted in hospitalization or death, Novavax said.
The vaccine was also well tolerated with few serious or serious adverse events in both the UK and South African trials, according to the company.
"We are very encouraged by the data showing that NVX-CoV2373 not only provided complete protection against the most severe forms of disease, but also dramatically reduced mild and moderate disease in both trials," said Stanley Erck, President and CEO. Novavax in the statement.
"Importantly, both studies confirmed efficacy against variant strains," said Erck.
Novavax's final analysis of the UK trial data "continues the stream of great news" the world has had on vaccines in recent months, said Michael Head, senior researcher in global health at the University of Southampton, in a statement distributed by the UK's Science Media Center in March.
"It is a remarkable achievement to have so many candidates already approved or showing such positive results before applying to regulators," said Head, who is not involved in the Novavax studies.
“A note of caution is the lower effectiveness observed against the worrying variant B.1,351, as first observed in South Africa.
This shows the impact that variants can have on the response to the pandemic and is therefore a strong argument to keep cases as low as possible while the burden of covid-19 is high, "he said.
"However, there is still a clear protective effect in the Novavax trial, and the data from all covid-19 vaccines suggest that they will be highly protective against serious diseases of all variants, which is still an excellent result."
- CNN's Maggie Fox contributed to this report.
novavaxCoronavirus Vaccine