Instant International
Written by: Xu Yi'an and Liang Kaiyi
2021-04-14 11:30
Last update date: 2021-04-14 11:40
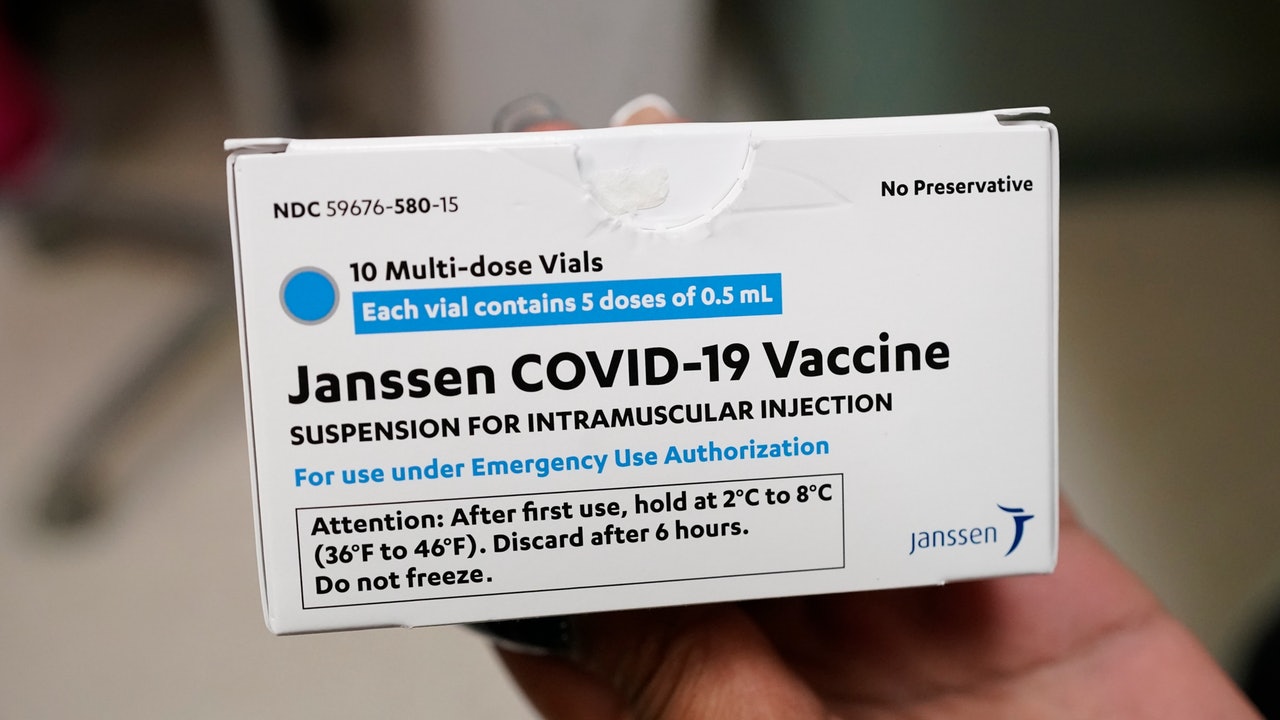
Someone in the United States developed thrombosis after being vaccinated with Johnson & Johnson's new coronavirus pneumonia (COVID-19) vaccine. The United States recommends to stop using it.
The European Medicines Agency (EMA) said on April 13 that it has noticed that the United States has suspended vaccination of this brand of vaccines, and that the inspections announced last week are continuing.
EMA issued a statement stating that it is not yet clear about the relationship between some incidents and vaccination, as well as the inspection actions announced by its safety committee last week.
The incidents and cases referred to by EMA refer to a rare blood clot in the United States after being vaccinated by Johnson & Johnson.
EMA issued a statement via e-mail on April 9 stating that among the cases it is examining, the four vaccinators with blood clots are Americans.
They also experienced low platelets after vaccination.
Three of the cases involved the production of vaccines by Janssen Pharmaceuticals, and the fourth person died during the clinical trial.
Janssen Pharmaceuticals is owned by Johnson & Johnson.
Johnson & Johnson said on April 13 that it is reviewing thrombosis cases with European health authorities and has decided to proactively postpone the launch of the vaccine in Europe.
EMA said that the safety committee is investigating thrombosis cases and will determine whether there is a need for regulatory action.
New crown pneumonia epidemic in the United States: The picture shows a student at Kent State University in the United States receiving the new crown pneumonia vaccine on April 8.
(AP)
Reuters quoted EU officials as saying that the EU is seeking clarification on Johnson & Johnson's "completely unexpected" announcement of the postponement of the vaccine.
He pointed out that Johnson & Johnson confirmed at the meeting on the 9th that the target is to deliver 55 million doses of vaccine within the scope of the contract to the EU by the end of June.
The EU spokesperson stated that the incident was being studied, but there was no comment.
The U.S. Food and Drug Administration (FDA) and the U.S. Centers for Disease Control and Prevention (CDC) issued a joint statement on April 13, stating that it is cautious to recommend suspension of the Johnson & Johnson vaccine.
The statement stated that as of April 12, six women in the United States had experienced thrombosis after being vaccinated by Johnson & Johnson.
New Coronary Pneumonia | Many people in the United States have thrombosis after being vaccinated by Johnson & Johnson. Authorities recommend suspension of use
New crown pneumonia | Johnson & Johnson postpones the launch of vaccine in Europe British media: EU seeks explanation
New Coronary Pneumonia | White House: Discontinuing Johnson & Johnson vaccine has little effect
New crown vaccine 丨 thrombosis after vaccination with Johnson & Johnson products, European Medicines Agency investigates 4 cases
Covid-19 vaccine Covid-19 U.S.