Social News
Written by: Zheng Cuibi
2021-05-15 14:37
Last update date: 2021-05-15 14:40
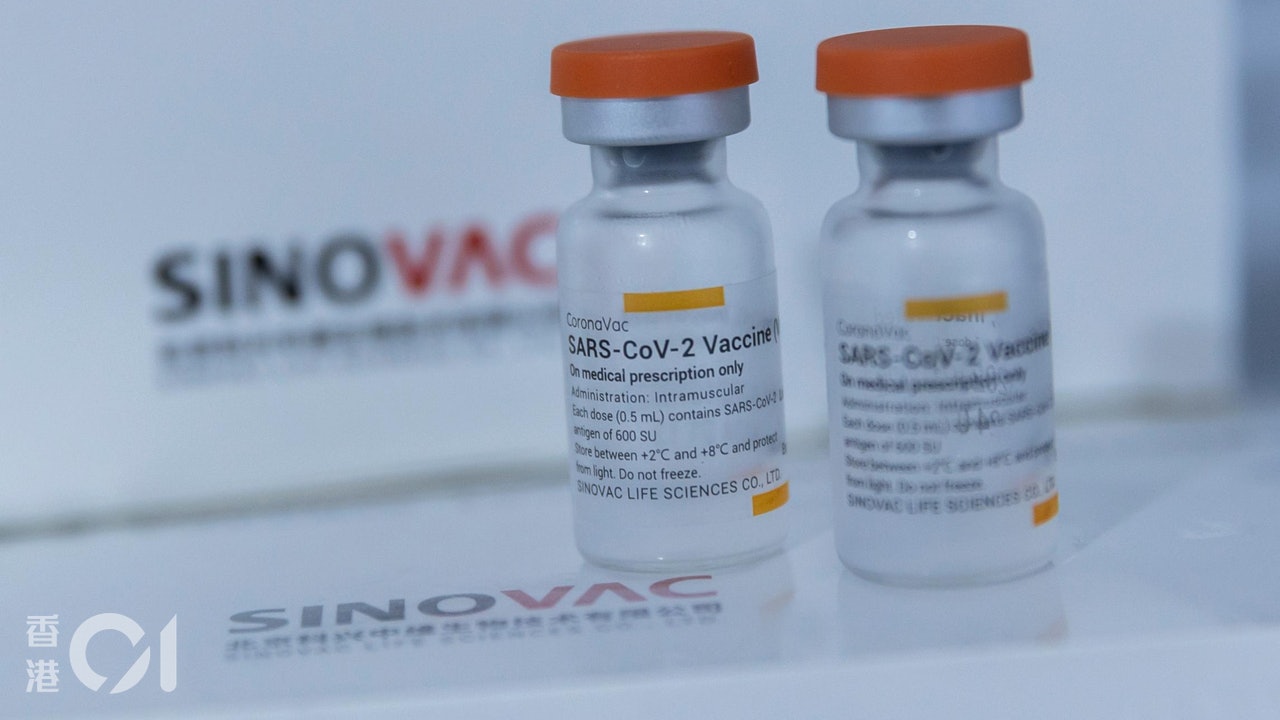
The World Health Organization announced on May 7 that it approves the emergency use authorization of the "Sinopharmaceutical Vaccine" developed in China, but for another "Kexing Vaccine" developed in the Mainland and used in Hong Kong, it will be decided this week.
However, I haven't seen any latest announcement from the WHO. "Hong Kong 01" asked the WHO about the situation and received a reply. It is still evaluating the emergency use authorization application for Kexing Vaccine. It will be announced after the evaluation is completed.
The WHO replied that the emergency use authorization application for Kexing vaccine is still being evaluated.
(Profile picture)
The WHO has stated that it is asking Kexing to provide more information and hopes to receive the information as soon as possible to make a decision. It is expected that an announcement will be made this week.
And Xu Shuchang, a government expert consultant and chair professor of the Department of Respiratory System at Chinese University, once pointed out that the WHO is expected to grant an emergency use authorization, and I believe it can be announced this week.
However, the WHO has not yet seen any latest announcement. "Hong Kong 01" asked the WHO about the situation. The WHO replied that it is still continuing to evaluate the emergency use authorization application of Kexing Vaccine. It will serve as a technical advisory group (technical advisory group). After the assessment is completed, the results will be announced to the public.
▼On May 8th, residents of Berjas Building will go home after completing the 21st quarantine▼
+5
+5
+5
There was no reply as to what information the WHO asked Kexing to submit.
In response to inquiries earlier, Kexing stated that it had not received a request from the WHO to submit more information.
Brazil will suspend the production of Kexing vaccines, saying that the supply of raw materials in China has encountered delays in customs clearance
The epidemic situation in the small towns of Indonesia and Brazil is stabilizing. Is Kexing vaccine effective?
China Kexing Vaccine|Indonesia study: 100% prevention of deaths in 7 days
The WHO said it asked Cohin for more information to decide whether to approve the emergency use of Coxing. Reply: Not received
01News
New Coronary Pneumonia New Coronary Vaccine Vaccine China Science and Technology World Health Organization