The pharmaceutical companies Pfizer and BioNTech announced on Thursday that they are developing a booster dose of their coronavirus vaccine to protect against the delta variant, which has already become predominant in the United States and threatens to cause another wave of infections due to its high transmissibility. .
[Vaccines protect against the dangerous delta variant of COVID-19. But it takes both doses]
Scientific studies have determined that both this vaccine and those of Moderna and Johnson & Johnson protect against the delta variant, although in the case of the first two it is necessary to receive both doses.
An additional injection has the potential to preserve "the highest levels" of immunization against all strains of the virus.
As reported, their preliminary studies on the efficacy and safety of this booster dose have shown good results and in the coming weeks they will request authorization from the Food and Drug Administration (FDA, for its acronym in English) for its use of emergency in the United States.
[Is it safe to mix COVID-19 vaccines? Can I reinforce the single dose from Johnson & Johnson with another from Moderna or Pfizer?]
"Evidence observed in the real world [compared to clinical studies] provided by the Israeli Ministry of Health shows that the efficacy of the vaccine decreases after six months," the pharmaceutical companies indicated in their statement.
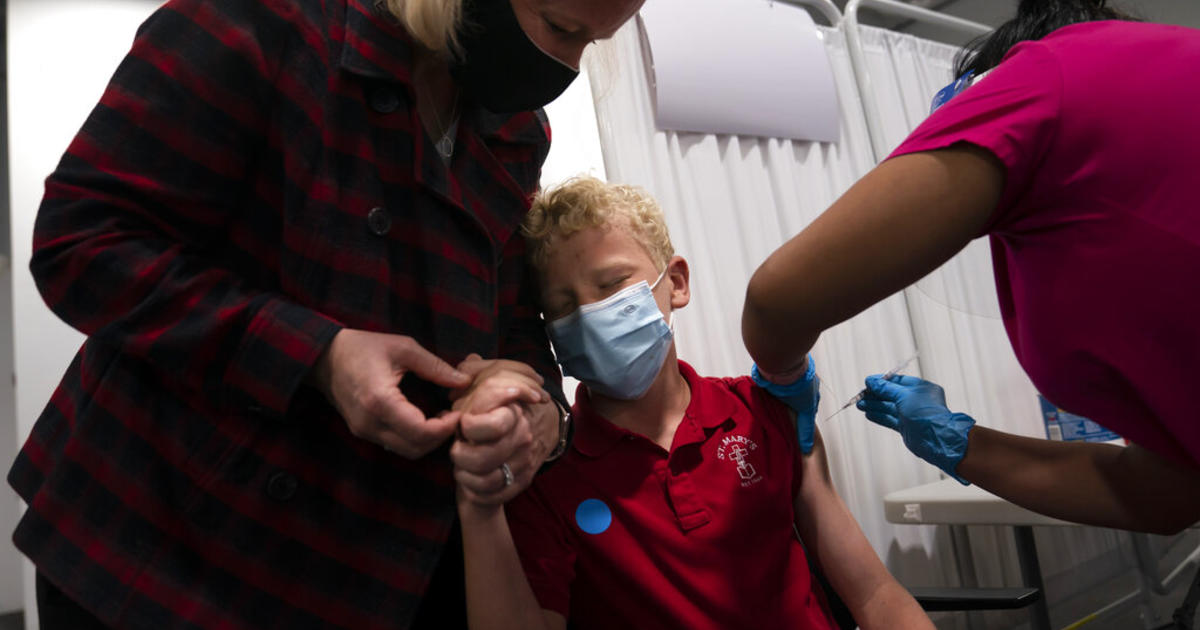
A 12-year-old boy receives his first dose of Pfizer's coronavirus vaccine on May 13, 2021.
"These findings coincide with the ongoing analysis of the phase 3 study" of the pharmaceutical companies, added Pfizer and BioNTech, indicating that, in their opinion (which should be corroborated by federal authorities), a third dose between six and 12 months after full vaccination with the two current doses.
[Axios Latino: Behind the multiple crises due to COVID-19 in Latin America]
Their initial studies show that an additional dose administered six months after the second injection increases the levels of antibodies between five and 10 times against the beta variant, the one initially detected in South Africa;
and also boosts defenses against the delta variant.
Pfizer's director of medical innovation, Mikael Dolsten, said in an interview with
The Associated Press
news agency
that the formal request to the US government will be made in August.
But FDA clearance would only be a first step
.
For Americans to receive a third dose, public health authorities would have to make that determination, explained Dr. William Schaffner, a vaccine expert at Vanderbilt University.
"The vaccines were designed so that we don't end up in the hospital" and they have continued to provide that protection against the delta variant.
Injecting a large number of people with a third dose would represent "a lot of effort, when we hardly try to get people to get the first dose."
Soon more information ...