news
Vaccine for corona
Vaccinate or wait?
Experts disagree on whether to give the elderly a third dose against Corona
Epidemic team members will once again discuss the initiative to give another dose to the most at-risk citizens, with three dilemmas dividing them: the degree of urgency, the need to wait for FDA regulatory approval, and the age from which to vaccinate.
Without sweeping consent and a clear recommendation, the move will not go ahead
Tags
Vaccine for corona
Corona virus
Meirav Cohen
Monday, July 26, 2021, 7:30 p.m.
Share on Facebook
Share on WhatsApp
Share on general
Share on general
Share on Twitter
Share on Email
0 comments
Members of the epidemic treatment team are expected to discuss next Wednesday the administration of a third vaccine dose to the elderly, after several discussions that ended without a sweeping decision.
However, in the shadow of the controversies on the subject, it seems that even this time unequivocal recommendations will not be accepted.
Committee members disagree on three issues: the level of urgency;
The need to wait for external regulatory approval;
And the practical question, that is: not only if - but also how to do it and in what age group exactly.
Read more on the subject
"She did not get vaccinated because she was afraid it would harm her pregnancy": a struggle for the life of the mother who fell ill in Corona
Bennett: "Immune refusers endanger us all";
Ministry of Health: The vaccine is 91% effective in preventing serious illness
5 tips that can save you a lot of money in car insurance
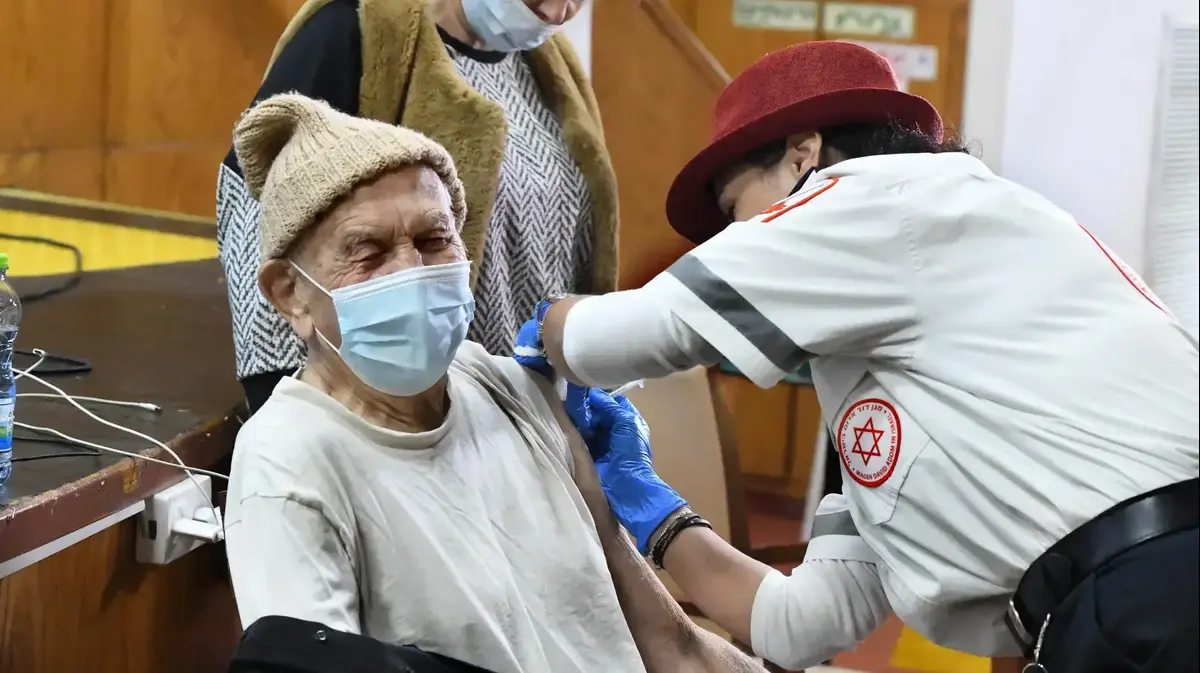
Immunization against Corona in the Mashen nursing home in Ramat Efal, January (Photo: Reuven Castro)
The first is the degree of necessity and urgency: the Ministry of Health would like to vaccinate seniors, at least those aged 70 and over who are at the highest risk - but some committee members think that in the current situation, where there are only 108 critically ill and 30 patients died in July, no Hourly justification for administering a third vaccine dose.
Hence the second dilemma: whether to vaccinate the elderly for the third time before approval by the United States Food and Drug Administration (FDA).
Prof. Galia Rahav explained to Walla!
Because the US regulatory authority is lagging behind Israel, so we did not wait for it to decide on the approval of a third vaccine for vaccine suppressants.
"Among vaccine-suppressed people, we only confirmed after clear findings in studies that showed that the effect of both doses is insufficient and they need another booster, especially in light of the outbreak," Prof. Rahav clarified.
More on Walla!
The first country in the world: Israel has started vaccinating in a third dose against Corona
To the full article
Rahav is currently working to obtain approval from the Helsinki Committee, which oversees human trials, for a third vaccine safety study in the elderly.
Once the approval is received, seniors at Sheba Hospital in Tel Hashomer will receive an additional dose.
"Only after I know that the third vaccine is safe and that there are no side effects, and in addition that it is of course effective and raises the level of antibodies in a beneficial way, only then will I give my consent to the third vaccine before FDA approval."
Therefore, at this stage it is expected to object to the committee discussion.
"Need to work responsibly"
The committee will be presented with a French study that shows an efficacy of over 30% for a third dose in immunosuppressants.
This is the latest research in the field, but because all the studies are immature, there is a need for Israeli studies that will clarify how to act correctly at this stage.
Other members of the committee emphasize that no additional vaccine dose should be approved for the entire elderly population, without the approval of the U.S. Authority or any other regulatory authority.
Prof. Hagai Levin, former chairman of the Public Health Association, stated that the procedures should be followed.
This is a very dangerous precedent, and must be passed responsibly, "he said.
Pushes for the third dose even harder.
Fire (Photo: Maariv)
The third dilemma facing the committee is pragmatic: if its members decide to give the green light to the third dose, they will have to decide at what age to do so - from age 65, or just age 75, which is the age group to which all dead from Corona have belonged in the past month.
At present, there is a majority for vaccinating the elderly among the committee members, and the director general of the Ministry of Health, Prof. Nachman Ash, is pushing harder for this, when he believes there is no need to wait for FDA approval. We did not attribute the elderly.
Share on Facebook
Share on WhatsApp
Share on general
Share on general
Share on Twitter
Share on Email
0 comments