news
World news
America
US medical officials want to delay booster release: "Want to collect more data"
Just before President Biden launched the third vaccination campaign, the head of the FDA and the CDC warned that more time was needed to approve the additional dose of all companies, except Pfizer, for which there is sufficient data.
However, they added that they would reach a decision soon.
"We always said we would keep track of science data," the White House said
Tags
U.S
FDA
cdc
Corona virus
Pfizer
News agencies
Friday, 03 September 2021, 23:30 Updated: 23:39
Share on Facebook
Share on WhatsApp
Share on general
Share on general
Share on Twitter
Share on Email
0 comments
US President Joe Biden addresses White House after completing ...
Prime Minister Bennett takes off returning to Israel after visiting the House ...
Attempted robbery in Haifa: Bank employee arrested after threatening ...
Prof. Zarka: PCR test for children will be valid for 4 days ...
Sgt. Barel Hadaria Shmueli shot to death on the Gaza border ...
Minister of Finance Lieberman before the discussions on the budget: a holiday ...
Terrorism in New Zealand: Police kill ISIS supporter stabbing people ...
Health Minister Horowitz in an interview with Walla!: We did ...
A crowd of young people preparing for the Panjoya Festival ...
Interception in the skies of Damascus as broadcast on television ...
A robot that cleans the sheets in the hotel
Safe Sex Campaign - A huge penis squirts on passers-by
In the video: The third vaccine against Corona opens to the entire population (Photo: Reuters)
Senior federal health officials have told the White House that they need more time and data from drug companies, other than Pfizer, to enable the administration of the third dose ("booster") against the corona virus to the entire U.S. population, the report said today (Friday). New York Times".
The head of the Food and Drug Administration (FDA), Dr. Janet Woodcock, and Dr. Rochelle Wolensky, who heads the Centers for Disease Control and Prevention (CDC), made it clear to the White House that their agencies could determine whether to recommend the extra dose in the coming weeks.
More on Walla!
USA: The FDA has given official approval for Pfizer's vaccine against Corona
To the full article
More on Walla!
USA: Authorities call on the public to get vaccinated in a third dose against Corona
Auditor's Report: Low enforcement and foreign considerations have hurt closures on red cities
Frozen shoulder?
Calcification?
Many problems that cause shoulder pain and one treatment that helps them all
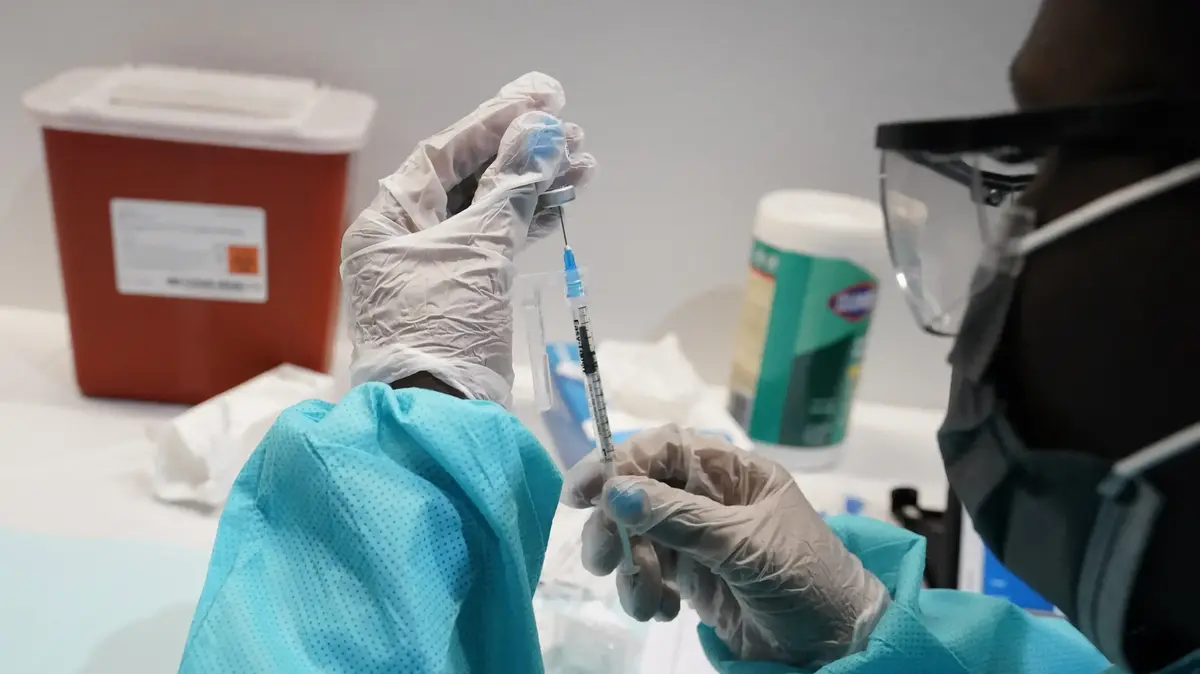
Waiting for more data (Photo: AP)
Both sources presented their recommendations in a meeting with Jeff Zintz, the Corona Headquarters Center at the White House.
Some people involved in the details of the meeting said it was not clear how Zintz responded, but he always insisted on listening to the experts' advice.
"We have always said we will monitor science data," White House spokesman Chris Migher said, adding that the administration is awaiting a "full review and approval" from the FDA and CDC.
"When the approval and recommendation is received," Migher said, "we will be ready to implement the program developed by the most senior doctors in our country so that we can be a few steps ahead of this virus."
Less than three weeks ago, US President Joe Biden said that provided the FDA approves, the third vaccination campaign will begin on September 20 for adults over the age of 65 and medical staff who received Pfizer's second injection at least eight months ago.
Share on Facebook
Share on WhatsApp
Share on general
Share on general
Share on Twitter
Share on Email
0 comments





/cloudfront-eu-central-1.images.arcpublishing.com/prisa/YIVHXZ4ULVEKTCBSNO56RKOWKI.jpg)








