U.S. media said on October 18 that the U.S. Food and Drug Administration (FDA) intends to allow U.S. citizens to use different brands of vaccines when they have completed two doses of new coronavirus pneumonia (COVID-19) vaccination.
In September, the US FDA granted emergency use authorization to vaccines jointly developed by the US pharmaceutical company Pfizer and the German biotech company (BioNTech) to allow them to be used as boosters.
People 65 years and older and high-risk groups can inject this vaccine as a booster.
According to the article, the FDA is expected to approve the vaccines produced by Moderna and Johnson & Johnson as boosters on the 20th, and the FDA may later allow them to be used for "groove needle" purposes.
For those who are vaccinated with Pfizer-BioNTech vaccine and Modena vaccine, two doses are the complete vaccination; Johnson & Johnson vaccine is a single-dose vaccine.
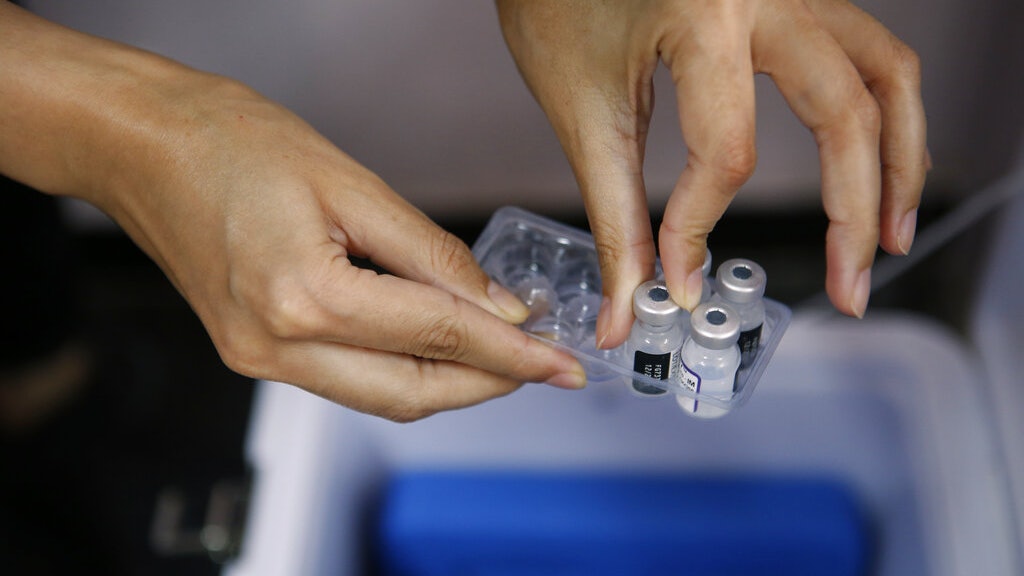
New crown pneumonia epidemic in the United States: The picture shows on October 5, medical staff holding a Pfizer vaccine in a Miami hospital.
(AP)
The newspaper quoted sources familiar with the plan as saying that Washington would not recommend individual brand vaccines and may say that the same vaccine should be used as an additional dose as much as possible.
The government may say that those who provide vaccination services may provide another brand of vaccine at their discretion.
This is a request from some state health officials for many weeks.
New crown pneumonia | US research: "Gutter needle" injection booster is safe and effective Singapore: People who have been vaccinated by Kexinghe Sinopharm may need to get booster new crown vaccine in the future | More than 1 billion people in the country have been injected with CDC recommends booster for certain groups of people
The US FDA declined to comment on the report.