The US Food and Drug Administration (FDA) announced on December 9 that it would extend the scope of the new coronary pneumonia vaccine booster commonly known as "Pfizer Vaccine" to 16 and 17-year-olds who received the first dose for at least 6 months.
This new type of coronavirus pneumonia (COVID-19) vaccine jointly developed by the US pharmaceutical company Pfizer (Pfizer) and the German biotech company (BioNTech) is commonly known as "Pfizer vaccine."
"Fubitai" is the commercial Chinese name of "Pfizer Vaccine" in China, Hong Kong and Macau. Its introduction and commercialization are undertaken by BioNTech's partner, Fosun Pharma.
After Pfizer and BioNTech announced the experimental data, the FDA decided to approve the emergency use authorization of the booster for this age group.
Experimental data shows that the booster can provide a high level of protection against the mutant virus Omicron.
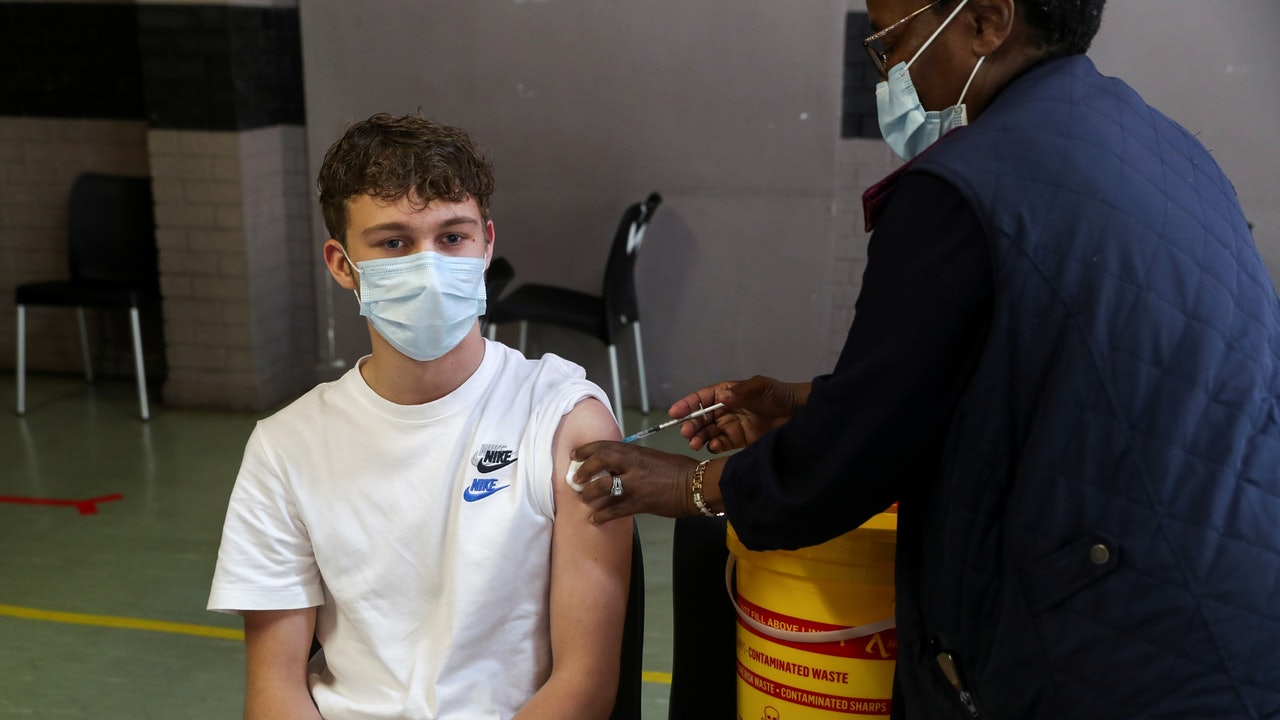
New crown pneumonia epidemic in the United States: The picture shows a student at Kent State University in the United States receiving the new crown pneumonia vaccine on April 8.
(AP)
Pfizer has announced that compared with Omicron's original virus, the effectiveness of two injections of Omicron is 25 times worse for Omicron.
However, the vaccination booster can greatly increase the level of antibodies to the original level of two injections, effectively neutralizing the Omicron virus.
After evaluating more data, the FDA believes that although it may cause myocarditis in young people, it believes that the benefits of vaccination still outweigh the risks.
Pfizer Vaccine | Pfizer: Experiments show that 3 doses of COVID-19 vaccine are effective in neutralizing Omicron Vaccine | Australia approved Pfizer’s new crown vaccine for children aged 5 to 11 years of Pfizer Vaccine | British media: UK and Pfizer have confidentiality terms Controversial arbitration does not disclose Pfizer vaccine | European Medicines Agency approves Pfizer vaccine for children aged 5 to 11
The FDA's decision still needs to be approved by the Centers for Disease Control and Prevention (CDC).
The latter approved on November 19 that all adults over 18 years of age in the United States can receive booster doses.