Pfizer withdrew its application for Food and Drug Administration (FDA) approval for use of its COVID-19 vaccine for children ages 6 months to 4 years, according to NBC News.
The company assured this Friday that it will wait until April to have the data from the studies it is conducting on the administration of three doses of the vaccine instead of two, because it believes that this "would provide a higher level of protection."
Pfizer had asked the FDA days ago to authorize the two-dose vaccination for children under five, and initially planned to present additional data on the booster later.
The booster dose only lasts four months
This Friday, a new study published by the Centers for Disease Prevention and Control (CDC) has also been revealed, which concludes that the booster doses of the Moderna and Pfizer-BioNTech vaccines lose "considerable" effectiveness about four months after administration.
Despite this, the reinforcement has offered significant protection to avoid the most serious symptoms and hospitalization for the omicron variant, according to The Washington Post.
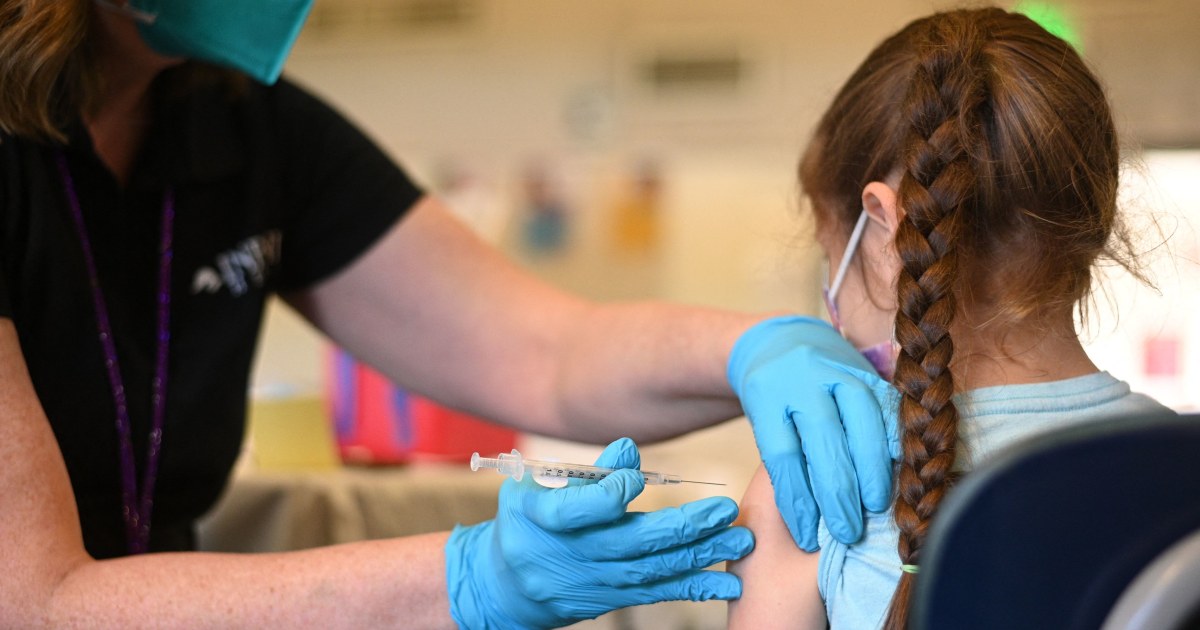
A nurse administers a pediatric dose of the COVID-19 vaccine to a girl at a vaccination clinic in the Sylmar neighborhood of Los Angeles, California, on January 19, 2022. Robyn Beck / AFP via Getty Images
The research, based on data from visits to hospitals and emergency rooms, suggests the need for additional doses, concluding that the booster dose offers 91% protection in the first two months against hospitalization, but drops to 78% at four months.