More than a million deaths from covid-19 in the US 4:38
(CNN) --
Pfizer and BioNTech announced Monday that three doses of their COVID-19 vaccine were safe and produced a strong immune response in children ages 6 months to 5 years.
This came after Moderna earlier announced that its two-dose vaccine was safe and produced a good immune response in young children.
The US Food and Drug Administration (FDA) is reviewing Moderna's application for emergency use authorization of its vaccine for this younger age group and will review Pfizer's soon as well.
Meanwhile, Covid-19 cases are on the rise across the country, driven by the highly contagious subvariant of omicron known as BA.2.12.1, according to new data from the US Centers for Disease Control and Prevention. USA (CDC).
5 keys to the data on deaths from covid-19 in the US
What should parents and caregivers know about the data obtained so far by Pfizer and Moderna?
What is the expected time frame for young children to be vaccinated?
And what can parents and caregivers do in the meantime to reduce their families' risk of infection?
advertising
To help answer these questions, I spoke with Dr. Leana Wen, a CNN medical analyst, emergency room specialist, and professor of health policy and management at George Washington University's Milken Institute School of Public Health.
She is also the author of "Lifelines: A Doctor's Journey in the Fight for Public Health" and the mother of two young children.
CNN:
Many parents have been eagerly awaiting the news of the vaccine for children under 5 years of age.
Are you optimistic based on what you've seen so far from the Pfizer studies?
Dr. Leana Wen:
Yes, I am.
To recap, Pfizer initially tested two 3-microgram doses in this younger age group (6 months to 5 years).
This dosage is one-tenth the adult dose (30 micrograms) and less than one-third the dose in children 5 to 11 years of age (10 micrograms).
Initial studies found that two doses at this lower amount were safe, but did not produce a sufficient immune response.
That's why Pfizer started testing a three-dose version of the vaccine, as other age groups have needed at least three doses to boost protection.
In fact, the FDA has authorized a third dose of Pfizer's vaccine for everyone age 5 and older, and the CDC has recommended this treatment.
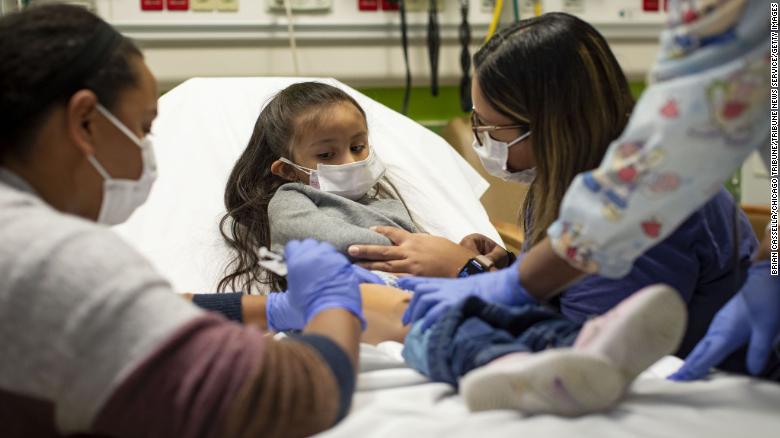
Trial participant Elena Rosales (center), 3, receives the Moderna vaccine against covid-19 from nurse Lela Lartey (left) with her mother, Mariaelena Lozano (right), by her side, on 7 December 2021, at Lurie Children's Hospital in Chicago.
Pfizer has just announced top-line data from a study of nearly 1,700 children who received a third dose during the period when the omicron variant was dominant.
Antibody levels measured one month after the third dose were similar to the response seen in young adults 16 to 25 years of age.
The company also reported that the vaccine was more than 80% effective against symptomatic covid-19 in children aged 6 months to 5 years.
However, these figures are considered mid-trial results and are not yet final.
Overall, these results seem promising to me.
I look forward to seeing the final results of the trial and Pfizer submitting their data for FDA review.
CNN:
How do Pfizer's results compare to Moderna's?
Wen:
It's hard to compare them head to head, so let me explain what Moderna's results showed.
Moderna tested a two-dose version of her vaccine.
The dose was 25 micrograms, which is a quarter of the dose of its adult version (100 micrograms).
The researchers found that the efficacy of the vaccine for children aged 6 months to 5 years was similar to that in the older age groups: specifically, that the vaccine is 51% effective in preventing symptomatic infection in children aged 6 months to less than 2 years and 37% effective in preventing symptoms in children aged 2 to 5 years.
Moderna's studies also found, similar to Pfizer's announcement, that its vaccine produced a robust antibody response in young children that was similar to that in older individuals.
The research team also found that the vaccine is safe.
I am optimistic about these results.
Moderna's results have already been submitted to the FDA, and the agency is currently reviewing this data.
CNN:
What's the schedule right now?
When can parents expect to vaccinate their children?
Wen:
The FDA just announced a meeting of its external advisory committee on June 15.
Agency officials said that day they will discuss Moderna's and Pfizer's applications for the youngest children.
Depending on the outcome of the meetings, if the advisers recommend the vaccines for authorization, the FDA could give the emergency use authorization immediately after meeting, and the CDC could meet and make its recommendation soon after.
With this type of schedule, parents may be able to start vaccinating their young children as early as the week of June 20.
If both vaccines are authorized, parents will have the choice between vaccinating their children with Pfizer's three-dose vaccine or Moderna's two-dose vaccine.
CNN:
Do you already know which vaccine you would give your children?
Wen:
Either vaccine, as long as they are licensed, whichever is available first.
I am confident in the thorough regulatory process and believe that both vaccines, if licensed by the FDA and recommended by the CDC, will be safe and effective.
I am looking forward to giving my young children, who are 2 and 4 years old, the excellent protection that everyone over 5 is entitled to right now.
CNN:
With Covid-19 cases on the rise, what can parents do in the meantime to protect their unvaccinated young children?
Wen:
Parents have to decide how important it is for them to continue to avoid the coronavirus.
Some may decide that it is less important, especially if the whole family has recently been infected with covid-19.
Others may decide that it is crucial, for example, if a child is immunosuppressed.
There are many methods that can help reduce risk.
Outdoor gatherings are still much safer than indoor gatherings.
Consider holding playdates, birthday parties, and other gatherings outdoors instead of indoors.
If you're hosting an event indoors, consider asking everyone to take a quick test at home before they arrive.
The use of masks in closed public spaces can also reduce the possibility of contracting the coronavirus.
covid-19vaccine against covid-19