The new findings on monkeypox 2:26
(CNN Spanish) --
Cases of monkeypox continue to rise around the world.
As of Tuesday, August 2, more than 25,000 cases of the disease had been confirmed, according to the US Centers for Disease Control and Prevention (CDC).
Meanwhile, in the United States, confirmed cases of monkeypox, which was recently declared a public health emergency of international concern by the World Health Organization (WHO), amounted to more than 6,300 on Tuesday.
As the disease progresses, attention is focused on various points, including the vaccines and treatment available so far.
On the one hand, in the case of vaccines, there are two available in the US, which are being distributed mainly in the states where the incidence of cases is highest: New York, California, Florida and Illinois.
Monkeypox Vaccines in the US: Where Are They Available and How to Get Them
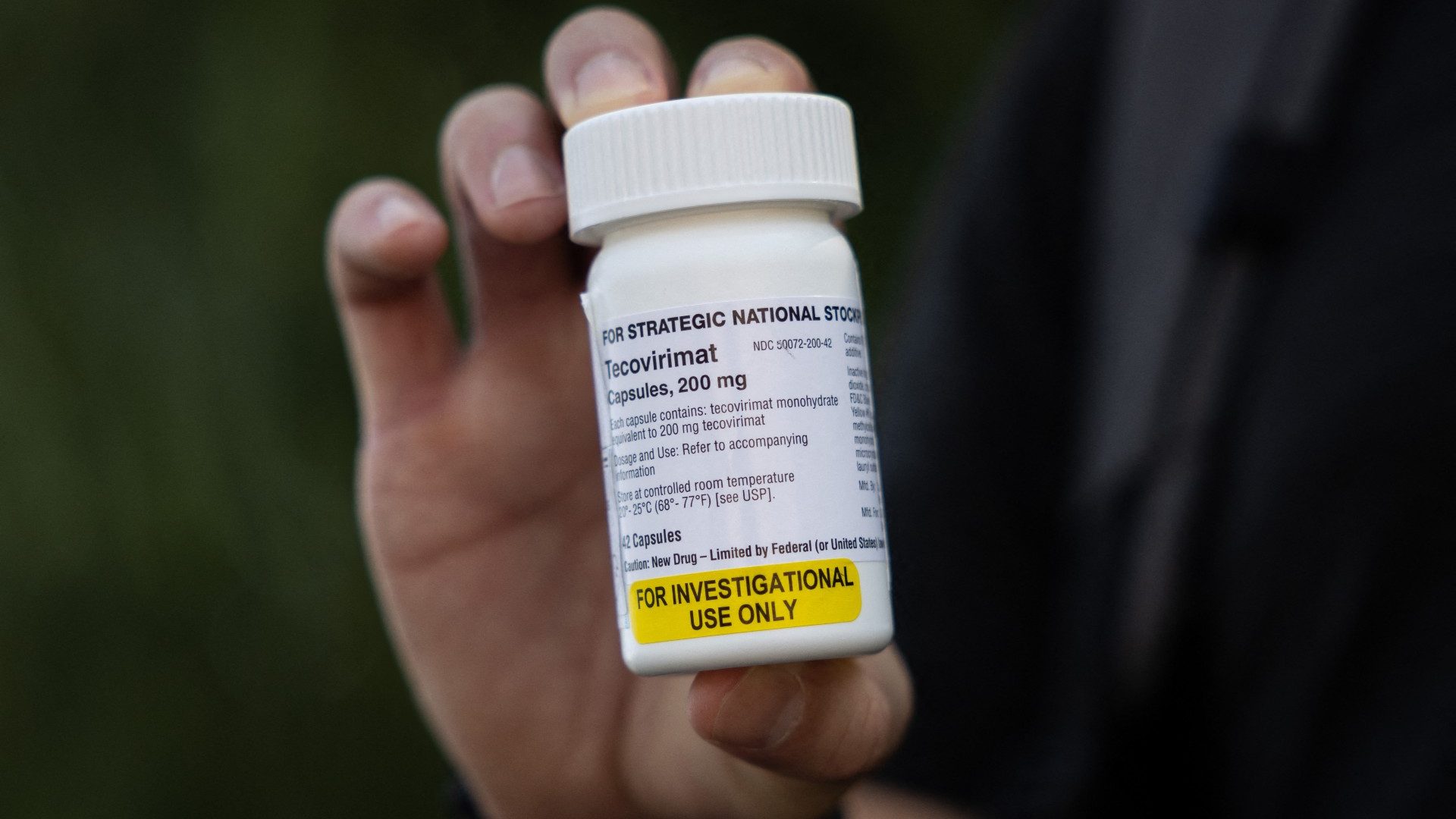
Tecovirimat, a monkeypox treatment
On the other hand, we have the issue of treatment, where there are four available (all experimental), but the most striking is Tecovirimat.
advertising
The US Food and Drug Administration (FDA) has not approved any specific therapies for the treatment of monkeypox.
However, the CDC made the antiviral drug Tecovirimat available during the current outbreak.
"Tecovirimat (also known as Tpoxx or ST-246) is approved by the FDA for the treatment of human smallpox caused by Variola virus in adults and children. However, its use for other orthopoxvirus infections, including monkeypox , is not FDA-approved.But the CDC has an Expanded Access Investigational New Drug (EA-IND) protocol that allows the use of Tecovirimat for primary or early empiric treatment of non-virus orthopoxvirus infections of smallpox, including monkeypox, in adults and children of all ages," the CDC says.
In other words, Tecovirimat (under the brand name Tpoxx) can be considered as an experimental treatment for severe monkeypox patients or those at high risk of severe disease.
Serious illness:
sepsis, inflammation of the brain, or other conditions that require hospitalization.
Risk of serious illness:
people who have weakened immune systems due to conditions such as HIV/AIDS, skin conditions such as eczema, children, pregnant women, and people with other complications such as a bacterial skin infection.
Others:
People with symptoms in particularly dangerous areas such as the eyes, mouth, genitals, or anus may also be considered for treatment.
Tecovirimat can be given as an oral pill or it can be given intravenously.
Difficulties to get it
The US lacked foresight in the face of monkeypox, says Dr. Huerta 1:40
A medical provider can request access to Tecovirimat by contacting their state Department of Health or the CDC.
However, doctors across the country suggest there are still significant hurdles to obtaining it, leaving some patients waiting days for shipments or traveling to find medical centers that can provide the product.
Doctors have outlined a series of steps to access the drug, including lab tests and consent forms.
"Just to put it in perspective, in my conversations with some of our treatment providers, between all the forms and administrative requirements, a patient's visit to start treatment can take anywhere from an hour and a half to three hours," said Dr. Mary Foote, medical director of the New York City Department of Health and Hygiene's Office of Preparedness and Response.
"Patients are scrambling to get this drug, even going out of town or out of state in some cases," said Dr. Peter Chin-Hong, an infectious disease physician at UCSF Health (San Francisco).
His hospital has received calls from patients all over California, as well as Colorado and even Canada and the UK, hoping to get treatment.
Dr. Anthony Fauci, the nation's top infectious disease expert, said the FDA and CDC are working to reduce the paperwork required.
This text includes information from Rocío Muñoz-Ledo, Neeraj G. Patel, Nadia Kounang, and Jen Christensen.
Tecovirimat Monkeypox




/cloudfront-eu-central-1.images.arcpublishing.com/prisa/YCO3PQLKSNBYHFVEPT47ZC3VGE.jpg)