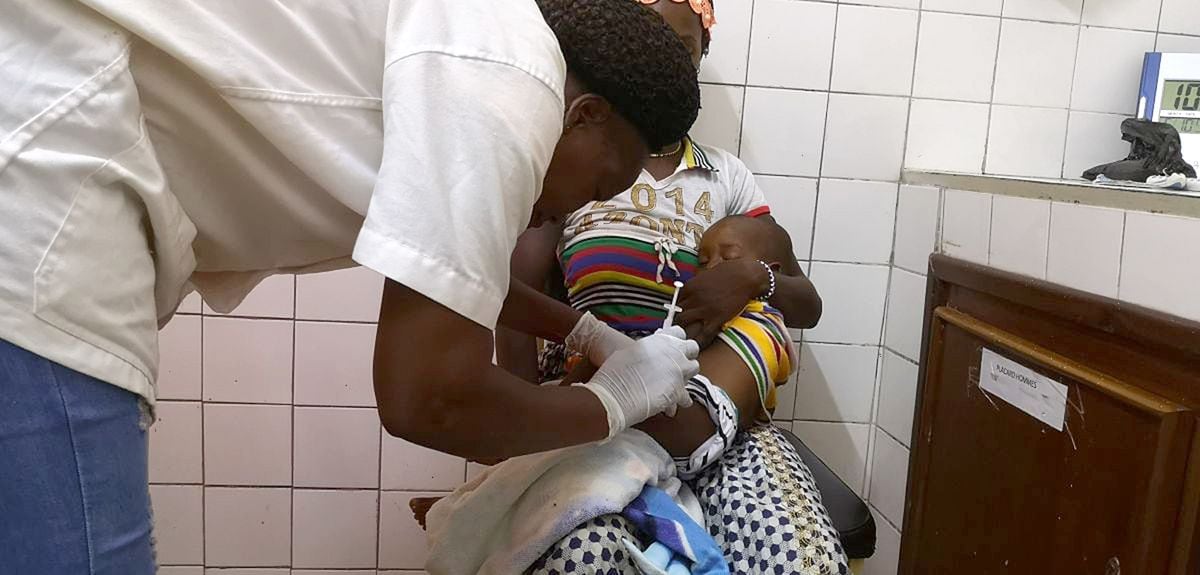
Researchers at the University of Oxford have reported the results of a phase 2b trial following the administration of a booster dose of their malaria vaccine candidate, R21/Matrix-M™.
This vaccine had previously shown 77% efficacy in a 2021 trial of young children in Burkina Faso, West Africa.
In their conclusions, published in
The Lancet Infectious Diseases
,
the team has shown that an extra dose per year, after a primary regimen of three doses, maintains a high efficacy against malaria, which complies with the technological roadmap set by the World Health Organization (WHO) of a vaccine with an efficacy of at least 75%.
According to the British university in a statement, the data correspond to a phase 2b, randomized, controlled and double-blind trial, carried out at the Nanoro Clinical Research Unit, of the Institute for Research in Health Sciences (IRSS, for its acronym in French) of Burkina Faso.
In it, a total of 450 children between the ages of five and 17 months were recruited in the Nanoro catchment area, of whom 409 received the booster.
The participants were randomly assigned to three groups, the first two received the R21/Matrix-M vaccine —with a low or high dose of adjuvant or substance that increases or modulates the immune response— as a booster, and the third received an anti-rabies vaccine as a booster group. control.
Each child received the same booster shot as their primary series of shots.
The doses were administered in June 2020, before the peak malaria season.
The investigators report a vaccine efficacy of 80% in the higher dose adjuvant group, and 70% in the lower dose group, during 12 months of follow-up.
The presence of antibodies was restored to levels similar to those of the primary vaccines 28 days after the administration of the booster doses.
No related serious adverse effects were observed.
Halidou Tinto, IRSS Regional Director at Nanoro and Principal Investigator of the trial, said: “It is fantastic to see such high efficacy again after a single booster dose.
We are currently part of a large Phase 3 trial, which aims to license this vaccine for widespread use next year."
For his part, Adrian Hill, director of the Jenner Institute at the University of Oxford and co-author of the article, expressed his satisfaction with the results showing that "a standard four-dose immunization regimen can now, for the first time, reach the high level of efficacy over two years that has been the goal of malaria vaccines for so many years.”
The R21/Matrix-M™ malaria vaccine candidate, developed by the University of Oxford, includes Novavax's proprietary saponin-based adjuvant Matrix-M and is licensed by the Serum Institute of India.
The trial is funded by the EDCTP2 program, supported by the European Union, as well as by the Welcome Trust and the NIHR Oxford Biomedical Research Centre.
And it has been extended for another two years, in order to assess whether more booster doses will be necessary to maintain high efficacy over time.
Results from the ongoing key Phase 3 trial to assess large-scale safety and efficacy in 4,800 children aged five to 36 months in four African countries are also expected later this year.
Malaria in numbers
The WHO estimates that malaria caused more than 640,000 deaths in 2020 and progress in reducing mortality from this disease has stalled in recent years.
Most deaths occur among children in Africa, where very high transmission rates are reported in many countries.
In 2020, some 240 million cases of clinical malaria were recorded.
Current malaria control measures include the use of insecticide-treated mosquito nets, fumigation, and seasonal malaria prevention, by administering monthly drugs to children at the time of peak transmission.
Until now, no vaccine has been licensed for widespread use, although development efforts have lasted more than 50 years.
In recent decades, more than 100 vaccine candidates have undergone clinical trials, but so far none have shown efficacy greater than the 75% predicted in the WHO malaria vaccine roadmap.
You can follow
MATERIA
on
,
and
, or sign up here to receive
our weekly newsletter
.