By Berkeley Lovelace Jr. -
NBC News
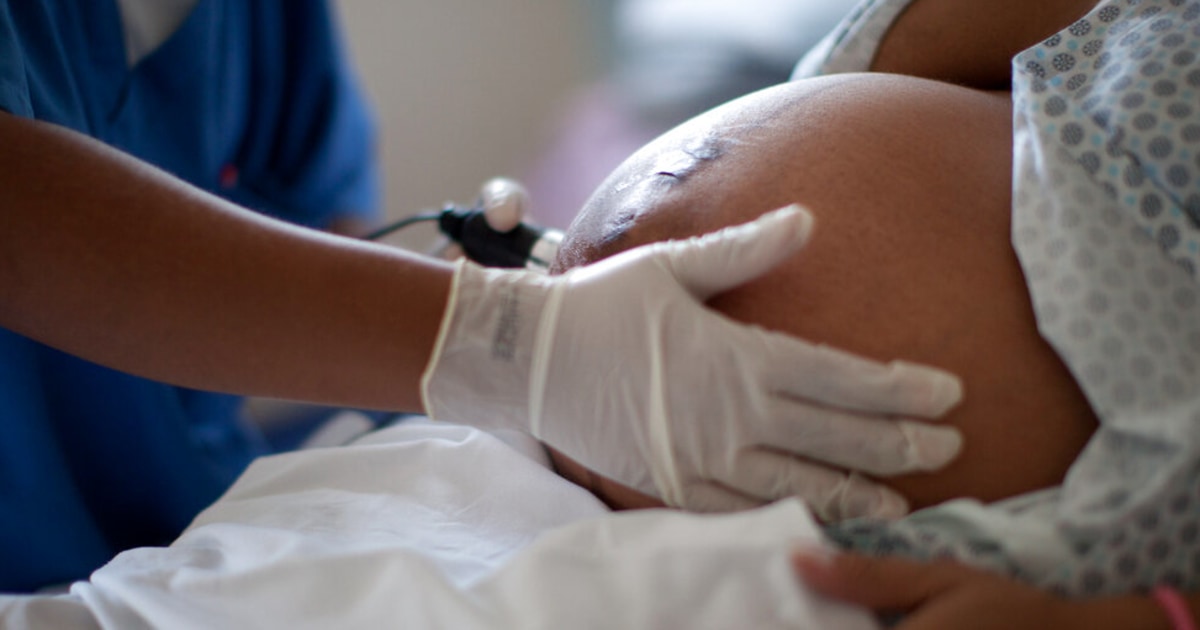
The advisory group of the Food and Drug Administration (FDA, for its acronym in English) approved this Wednesday to withdraw from the market a drug intended to prevent premature births, noting that its effectiveness is doubtful.
In a 14-1 vote, the recommendation from the federal agency's Obstetric, Reproductive and Urologic Drugs Advisory Committee closed a three-day meeting on clinical trials supporting Makena, the only drug approved in the United States to prevent childbirth. before term.
A pregnant woman during a check-up before giving birth. Felipe Dana / AP
The meeting included emotional testimony, including from advocates who said removing the drug could exacerbate racial disparities in maternal health.
The panel voted on three questions: whether the drug is effective, whether trial data support its approval, and whether it should stay on the market.
The panel voted no on each of the questions.