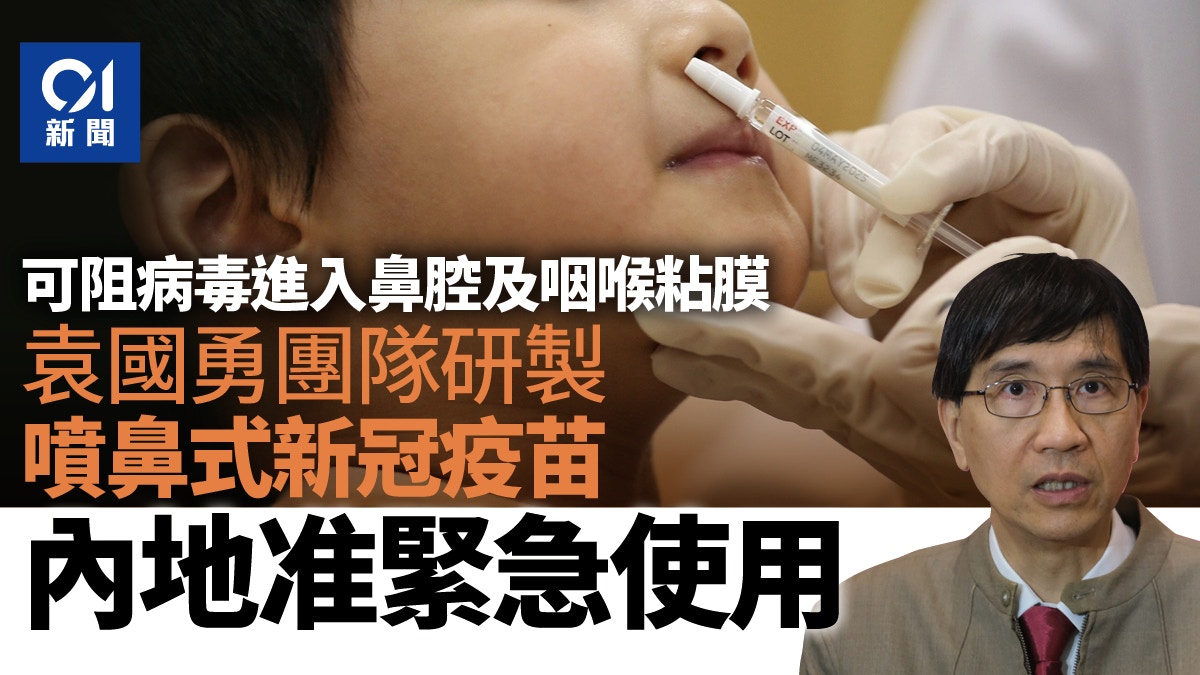
The nasal spray COVID-19 vaccine developed by the team of Chair Professor Yuan Guoyong of the Department of Microbiology of the University of Hong Kong, Beijing Wantai Biotech and Xiamen University has been approved for emergency use in the Mainland.
Beijing Wantai Bio announced that the nasal spray vaccine has been approved by the State Drug Administration for emergency use after being proposed by the National Health and Health Commission.
Yuan Guoyong said that unlike the existing injectable vaccines, the new nasal spray vaccine can prevent the virus from entering the nasal cavity and throat mucous membranes; those who have been vaccinated with inactivated vaccines before receiving the nasal spray vaccine can protect 80% of the Omicron variant virus , 55% protection even without vaccination.
The University of Hong Kong stated that Wantai aims to produce 200 million doses of vaccines in the next six months, but whether it will be used in Hong Kong still awaits the approval of the Hong Kong government.
Good protection against Omicron virus strains
Beijing Wantai Biological announced that the nasal spray COVID-19 vaccine has been approved for emergency use by the State Drug Administration. On November 10 last year, it was approved by the South African Food and Drug Administration for the Phase III clinical trial, and was subsequently issued by the relevant departments of the Philippines, Colombia, and Vietnam for the Phase III clinical trial.
The announcement pointed out that the main data analysis of the third phase of clinical trials of the vaccine was completed in October this year. The data showed that no matter it is used for basic immunization of people without immunization history or sequential booster immunization of people with immunization history, they are all resistant to Omicron variant virus strains. Good protective effect, the protective effect of people over 60 years old is not weaker than that of people aged 18 to 59, and it has good safety.
The University of Hong Kong is developing a nasal vaccine for the new crown.
(profile picture)
Yuan Guoyong: The vaccine prevents the virus from entering the nasal cavity and throat mucosa
Yuan Guoyong said that the nasal spray vaccine can prevent the virus from entering the nasal cavity and throat mucous membrane, which is different from the injectable vaccine, which can make the body produce blood neutralizing antibodies and T cells to resist the virus, but it will not be produced in the nasal cavity and throat mucous membrane.
Chen Honglin, a member of the team and a professor of the Department of Microbiology at the University of Hong Kong, added that the nasal spray vaccine is a better supplement to the current vaccination, and it is more acceptable to children and other people who are afraid of injections. It is suitable for high-risk groups such as the elderly and chronically ill people to be vaccinated first.
Hong Kong university.
(profile picture)
Up to 80% protection against Omicron variant virus
The University of Hong Kong pointed out that the three phases of clinical trials have confirmed that the vaccine is safe for humans, and in the third phase of the trial, it has been confirmed that if people who have been vaccinated with the inactivated vaccine receive the nasal spray vaccine, their protection against the Omicron variant virus is possible. It reaches 80%, and the protection power of unvaccinated people is also 55%.
The University of Hong Kong continued to say that the research team is updating the vaccine for the new variant virus.
Wantai will produce 2 million doses in the next six months.
The University of Hong Kong stated that it has yet to be approved by the Hong Kong government for use in Hong Kong.
▼December 3, Valley Needles, Civil Service Bureau Mall▼
+4
In a written response to questions from the Legislative Council last Wednesday (November 30), Lu Chongmao of the Medical and Health Bureau mentioned that the vaccine was approved by the Medical and Health Research Fund of 20 million yuan for the first and second phases of clinical testing for safety.
According to the bureau, the first phase of clinical trials has been completed in 29 volunteers aged 18 to 55, and no major adverse reactions were found, reflecting that the vaccine is safe for human use, and the vaccine is also used as a booster in the second phase The clinical test has been carried out on more than 100 volunteers aged 18 to 75. The research team is now collecting immune data and analysis, which is expected to be completed in the first quarter of next year.
Strong Inspection Building|An increase of 56 places in Tuen Mun District, the most in Shatin City One, Mid-Levels Yuyuan on the list, Tseung Kwan O, Sunrise Lohas Park Marini, and Aidiewan in the Eastern District. The government sent 50,000 quick test kits Epidemic|An increase of 9,508 confirmed hospitalizations The number of people is about 3,000, which hit a new high after the rebound.
Latest | 9,508 new confirmed cases; Hong Kong University develops a nasal vaccine; the mainland takes the lead in using compulsory testing | 51 places on the list Tsuen Wan District is the most involved in Discovery Park, Allway Gardens, Park Island, etc.