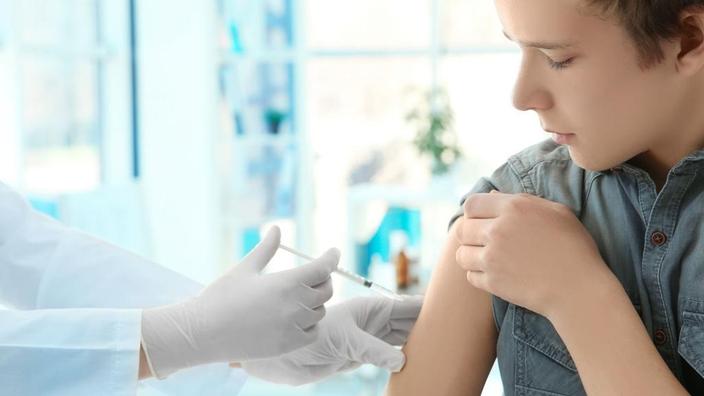
After Canada and the United States, it is the turn of the European Union to give the green light to the vaccination of adolescents against Covid.
Pfizer / BioNTech's Comirnaty vaccine, already authorized from the age of 16, is now also authorized for 12-15 year olds.
To discover
Covid-19: vaccination as the only remedy
Read also: Covid-19: is the epidemic finally behind us?
This is hardly a surprise, as the results presented by Pfizer on this age group were good. According to the study published in the New England Journal of Medicine, the safety of the vaccine in adolescents as well as its effectiveness are the same, or even better than those observed in young adults. The authors compared the results obtained in 1,131 adolescents aged 12 to 15 years who received two doses of the vaccine (in the same quantities and vaccination schedule as in adults), and in 1,129 who received a placebo (saline solution), as well. than with data collected from 16-25 year-olds vaccinated on the same scheme.
As in adults, the main side effects are mild reactions of immunogenicity (expected signs of the immune reaction): pain at the injection site, fatigue, headaches, etc. They are stronger on the second injection.
No case of Covid was diagnosed in the vaccinated (against 16 in the placebo group) and the production of neutralizing antibodies is 1.8 times greater than that measured in young adults.
On the other hand, the data do not allow to comment on the efficacy against transmission (especially as possible asymptomatic infections were not investigated), nor on the duration of protection (the trial started in July 2020).
Safety and efficiency
Canada was the first country in the world to authorize the Pfizer vaccine for adolescents on May 5, but only opened the doors to medical centers for them last weekend.
In the United States, where the emergency authorization was given on May 10, the vaccination of adolescents began on the 13th and more than 2.5 million 12-15 year olds have already received at least one dose!
Read also: Covid-19: the five major challenges of the vaccine campaign
Others have started trials in pediatric populations. Moderna in particular has presented excellent safety and efficacy data after a trial of 3,700 adolescents aged 12 to 18, and plans to file applications for authorization with regulators, including the US FDA and the United States. 'European EMA, "early June". Pfizer also launched trials in children aged 6 months to 11 years in March, and aims to recruit more than 4,600 participants in the United States and Europe for expected results in early 2022.
France will still have to wait for the opinion of the High Authority for Health, expected "
in the course of next week
". It will then not only be a question of taking into account the safety and efficacy of the vaccine, but also the vaccine strategy and the availability of doses, in France and around the world.