Enlarge image
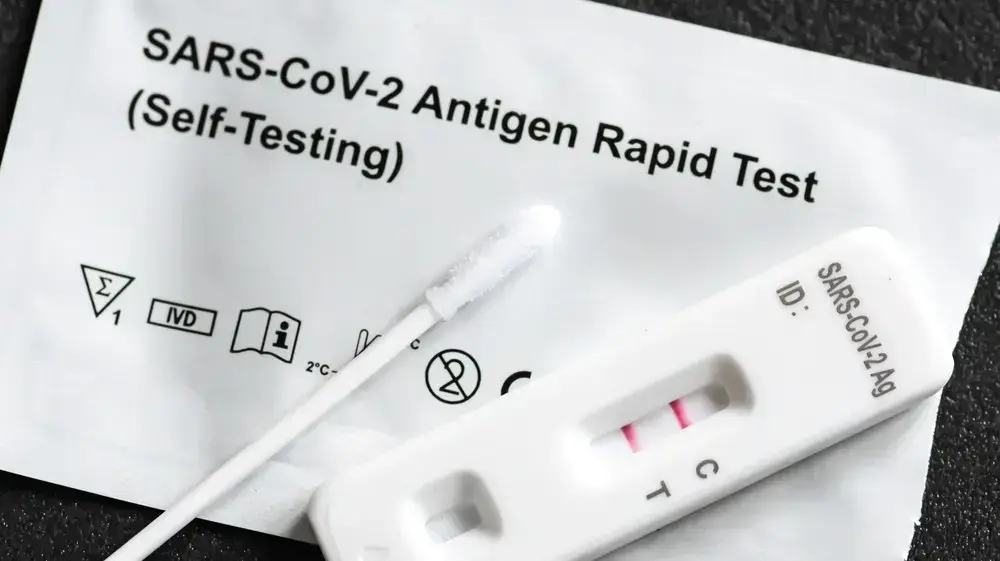
Do rapid tests reliably recognize Omikron?
(icon picture)
Photo: Sebastian Gollnow / dpa
The US Food and Drug Administration (FDA) warned in December that antigen tests might not detect omicron infection as reliably as infection with earlier variants of the coronavirus.
Two smaller studies also raised doubts as to whether rapid tests are suitable for detecting omicrons.
The Paul Ehrlich Institute for Vaccines and Biomedical Drugs (PEI) has now followed up on this information and examined antigen tests used in Germany.
The result: The PEI did not find any evidence of reduced sensitivity of rapid antigen tests to Omikron.
It is well known that rapid tests per se are not as reliable as PCR tests.
Their sensitivity, i.e. the probability with which they recognize infected people as such, is lower.
The time at which the sample was taken, the viral load of the infected person and the quality of the sample itself also play a role.
At the beginning of an infection - when the infected person is most contagious - they do not work as well as towards the end of the disease.
Not only the FDA, but also a study by the Ludwigs-Maximilians-University in Munich (LMU) found out that the antigen tests at Omikron are even less reliable.
In the LMU investigation, eight out of nine tested rapid antigen tests failed in the laboratory experiments.
The PEI now checked 20 randomly selected tests for professional use.
The research found that the tests were just as sensitive to omicron as to the delta variant and wild-type coronavirus.
The institute assumes that the results of the 20 tests can also be applied to other tests with a similar design.
According to the "Ärzteblatt", the PEI announced the results on Thursday in a virtual press meeting.
However, the study also revealed that, according to the manufacturer, at least 43 tests use binding regions that overlap with an omicron mutation region.
This means that the antibodies used in the tests could possibly bind less well to the omicron viruses - and thus not detect them as well.
The affected manufacturers now have until mid-April to submit information about the antibodies used in the tests and to prove that they can also recognize a mutated spike protein.
The Federal Institute for Drugs and Medical Devices (BfArM) lists antigen tests on its website that meet the minimum criteria valid in Germany: According to this, a test must have a sensitivity of at least 80 percent, i.e. detect at least 80 out of 100 infected people.
BfArM list updated - consumers should check
However, the BfArM list is based on the manufacturer's information.
The PEI also checks random antigen tests and checks whether the manufacturer's information is correct.
As a result of the latest findings, the institute has now adjusted its list and introduced a new column: »Omicron detection according to the PEI bridging test«, it now says.
If a »yes« is noted there for the corresponding test, this means that the omicron mutation does not influence the result.
Consumers should therefore find out which tests meet the minimum criteria before purchasing a self-test either from the BfArM or on the PEI website.
cry