By Aria Bendix —
NBC News
There are no more monoclonal antibody treatments for COVID-19 available in the United States: the Food and Drug Administration (FDA) on Wednesday rescinded the authorization of Bebtelovimab, a drug that was previously given to patients at high risk of developing the illness.
In the past two years, the FDA had authorized six monoclonal antibody treatments for the coronavirus, but successive subvariants of omicron made the drugs less effective, so the FDA gradually revoked each of those authorizations.
Bebtelovimab, made by Eli Lilly, was the last one standing.
According to the FDA announcement, the drug “was not expected to neutralize the
BQ.1 and BQ.1.1 subvariants.
omicron”, which together now account for the majority of new infections reported in the US, about 62%, according to data from the Centers for Disease Control and Prevention (CDC, in English).
They announce that an experimental vaccine against HIV is showing good results against the virus
Dec 2, 202200:30
“The big problem is that monoclonal antibodies bind to a very small part of the virus.
As the virus changes, we find ourselves in a situation where we have lost them all because they no longer bind to the virus,” says Arturo Casadevall, a professor of medicine at Johns Hopkins School of Medicine.
[Authorities Investigate Accidental Overdose of 10-Month-Old Baby with Fentanyl in San Francisco]
Paxlovid
has become the gold standard
for most people at high risk of severe COVID-19, as it remains effective against new variants and is easy to administer (it is a three-pill series that taken twice a day for five days).
Bebtelovimab
,
on the other hand, was an intravenous infusion that lasted about an hour.
But doctors used to recommend the monoclonal antibody to people taking certain immunosuppressive drugs, such as cancer patients or transplant recipients, because Paxlovid can negatively interact with several of these drugs.
This experimental drug has shown its effectiveness against Alzheimer's
Dec 1, 202200:24
Rodney Rohde, chair of the Texas State University Clinical Laboratory Sciences Program, said he is concerned about
how immunocompromised patients will fare with fewer treatment options.
[A New Alzheimer's Drug Appears To Slow The Disease But With Significant Risks, Study Finds]
“There are still segments of the population that probably have very little protection,” he said, “we are concerned about the last push that the virus may have this winter, and looking forward to spring, if we see higher mortality or hospital beds fill up ”.
In the spring, when the BA.2 omicron subvariant was dominant, research by Raymund Reasonable, an infectious disease specialist at the Mayo Clinic in Minnesota, showed that
Bebtelovimab was as effective as Paxlovid
in preventing severe disease among patients with high risk.
However, Reasonable said his hospital switched to Paxlovid as the main treatment over the summer as the new subvariants began to spread.
About two months ago, he said, doctors began asking patients who received bebtelovimab to report whether their symptoms had not improved in a day or two.
When the FDA revoked the treatment's authorization, the Mayo Clinic had already stopped giving it, Reasonable said, as doctors assumed it was no longer effective against BQ.1 and BQ.1.1.
Los Angeles registers 64% of children's beds occupied due to the 'tripledemic'
Nov 29, 202202:03
Reasonable said that Mayo Clinic patients who are at high risk of severe COVID-19 but cannot take Paxlovid are now offered the antiviral drug remdesivir at the infusion center where the hospital previously administered the monoclonal antibodies.
But remdesivir infusions are given over three days, so patients need multiple appointments.
Another option for immunosuppressed people is
convalescent plasma,
which is obtained from blood donated by people who have already recovered from the coronavirus.
Casadevall said that convalescent plasma is an effective alternative to monoclonal antibodies, but it is more complicated to administer and monitor.
“It has thousands of different antibodies, so convalescent plasma has a great amplitude that you don't find in monoclonals,” he said.
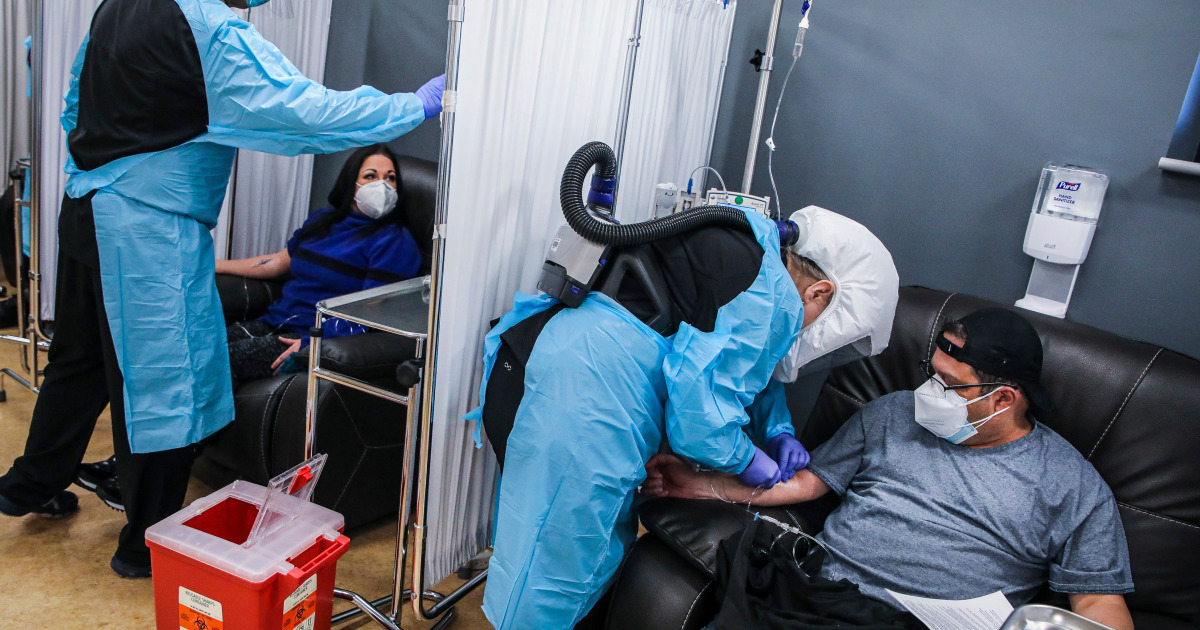
Dr. Sandeep Jubbal, left, and nurse Sheryl Tribou treat patients with COVID-19, at UMass Memorial Health in Worcester, Massachusetts, on Dec. 23, 2021.Erik Clark/Boston Globe via Getty Images
However, pharmaceutical companies have not given up on the promise of new monoclonal antibody drugs.
“We continue to strongly believe that [monoclonal antibodies] have a critical role in the ongoing fight against COVID-19, especially in high-risk individuals,” said a spokesperson for Vir Biotechnology, the company behind a monoclonal antibody. called Sotrovimab.
An Eli Lilly spokesman said the company is seeking and evaluating monoclonal antibody candidates.
AstraZeneca, for its part, said it is developing a new antibody cocktail that it hopes to have ready by the end of next year.
The FDA approved AstraZeneca's monoclonal antibody drug Evusheld in December 2021, not as a treatment for the coronavirus, but as a way to prevent infection in immunosuppressed people who may not develop a strong antibody response to vaccines.
But in October, the FDA announced that Evusheld might not be effective against circulating variants, and Reasonable said his hospital is preparing to have its authorization revoked soon.
Pediatric units are at the limit and doctors beg parents not to make "the biggest mistake"
Nov 29, 202201:51
"Looking at the distribution of variants today, Evusheld is effective maybe 30% of the time," he said.
Casadevall said that new monoclonal antibody drugs are still worth pursuing, as they have been shown to be safe and effective against COVID-19.
"I wouldn't give up on something like that," he said, "I think it's possible to find antibodies that are active with the other variants."