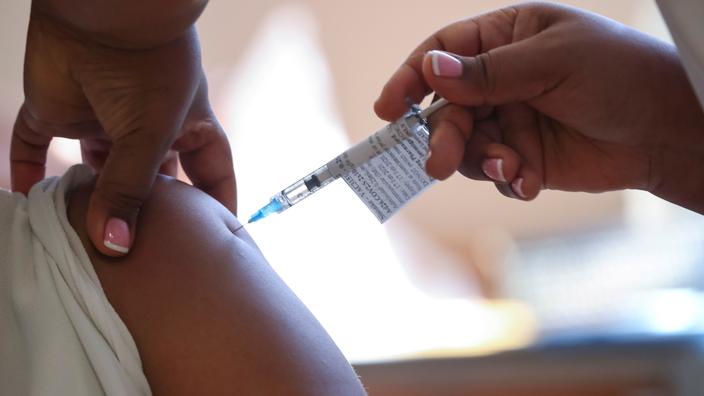
South Africa announced on Monday the first step to endow the continent with a production capacity for anti-Covid vaccines, but it will take time to materialize the project and while waiting
"people continue to die"
, pointed out guard President Cyril Ramaphosa.
Read also: Where is vaccination in Africa?
The South African leader, spearheading the fight for the temporary lifting of intellectual property on anti-Covid vaccines and slayer of vaccine inequality, had this terrible sentence on Monday to justify the creation of a regional production capacity :
“We can see that we cannot count on vaccines that are manufactured outside Africa because they never come. They never arrive on time and people keep dying ”
. He was speaking from South Africa at a World Health Organization press briefing in Geneva on the project.
His country accounts for more than 35% of the total Covid-19 cases recorded in Africa and is currently suffering from a third wave of massive infections, and like other developing countries he is seeing vaccines go to rich countries or in countries which, like India, produce them on a large scale.
"Technology transfer center"
Only 2% of the population of the African continent has had at least one dose, said President Ramaphosa, when the United States or Europe are targeting 70% of the population fully immune in the coming months. Monday's announcement must eventually remedy this imbalance. This involves setting up a
"technology transfer center"
for anti-Covid messenger RNA vaccines, which have proved to be extremely effective like the sera from Pfizer-BioNTech or Moderna and which also seem more easily adaptable to new variants, than other vaccines of different technology.
"This technology hub will allow a rapid response for the development of new vaccines, whether for variants of Covid 19 or future pathogens on the African continent and for the benefit of the whole world,"
said French President Emmanuel Macron, in a report. recorded message.
He pledged, in addition to the donation of vaccines, to help Africa build up its own production capacity.
The project is led by a South African consortium made up of biotechnology companies Biovac and Afrigen Biologics and Vaccines, a network of universities and African Centers for Disease Control.
Read also: Research, clinical trials, quality controls: how do you make a vaccine?
WHO has already established such centers with the aim of boosting the global production of influenza vaccines.
In fact, these centers provide know-how and training to local manufacturers where the technology taught is available on an industrial scale.
Manufacturers in low- and middle-income countries who are interested can find the necessary training and licenses here.
WHO and its partners bring their know-how in production, quality control and intellectual rights to accelerate the diffusion of technologies.
Vaccines produced no earlier than 9 to 12 months
In the South African configuration, Biovac will be the developer, Afrigen the manufacturer and the network of universities will contribute knowledge in messenger RNA, with technical support from CDC Africa. WHO has stressed that the South African hub has capacity available.
But as the head of the WHO Tedros Adhanom Ghebreyesus recalled:
“This is an important step which will yield results in the medium term. In the short term we must do everything in our power to increase the production and equitable distribution of vaccines through Covax ”
. What timetable? WHO Chief Scientific Officer Dr Soumya Swaminathan first outlined an optimistic scenario, where everything goes as planned, which would see
“vaccines produced in South Africa in the next 9 to 12 months”,
using already proven and approved processes. If new methods of using messenger RNA were needed, more time would be needed, particularly because of the necessary clinical trials.