Dr. Huerta: Hydrogen peroxide and iodine do not end with covid-19 1:36
(KHN) -
One day after testing positive for COVID-19 in June, Miranda Kelly was sick enough to be scared.
At 44, with diabetes and hypertension, Kelly, a certified nursing assistant, had trouble breathing - symptoms severe enough to send her to the ER.
When her husband Joe, 46, also caught the virus, she really worried, especially about her five teenage children at home: "I thought: 'I hope to God we don't end up with respirators. We have children. Who goes to raise these children? "
But the Kellys, who live in Seattle, had agreed right after their diagnosis to join a clinical trial at the nearby Fred Hutch cancer research center, part of an international effort to test an antiviral treatment that could stop COVID in its early stages.
The next day, the couple were taking four pills, twice a day.
Although they weren't told whether they had received an active drug or a placebo, within a week, they said, their symptoms had improved.
Within two weeks, they had recovered.
"I don't know if we got the treatment, but I feel like we did," Miranda Kelly said.
"To have all these underlying conditions, I felt that the recovery was very fast."
The Kellys play a role in developing what could well be the world's next chance to stop COVID: a short-term regimen of daily pills that can fight the virus at an early stage after diagnosis and possibly prevent it from symptoms develop after exposure.
advertising
Pfizer CEO says they will be ready in a matter of days to request approval of a covid-19 vaccine for children
"Oral antivirals have the potential not only to reduce the duration of the COVID-19 syndrome, but also to limit transmission to people at home if you are sick," said Timothy Sheahan, a virologist at the University of North Carolina. -Chapel Hill who has helped promote these therapies.
Antivirals are already essential treatments for other viral infections, such as hepatitis C and HIV.
One of the best known is Tamiflu, the widely prescribed pill that can shorten the duration of the flu and reduce the risk of hospitalization if given quickly.
The drugs, developed to treat and prevent viral infections in people and animals, work differently depending on the type.
But they can be designed to boost the immune system to fight infection, block receptors so viruses cannot enter healthy cells, or reduce the amount of active virus in the body.
At least three promising antivirals against COVID-19 are being tested in clinical trials, with results expected as early as late fall or winter, said Carl Dieffenbach, director of the AIDS Division at the National Institute of Allergy and Infectious Diseases. , which is overseeing the development of antivirals.
The antiviral molnupiravir, a promise in the treatment against the coronavirus?
"I think that in the coming months we will have answers about the capacity of these pills," Dieffenbach said.
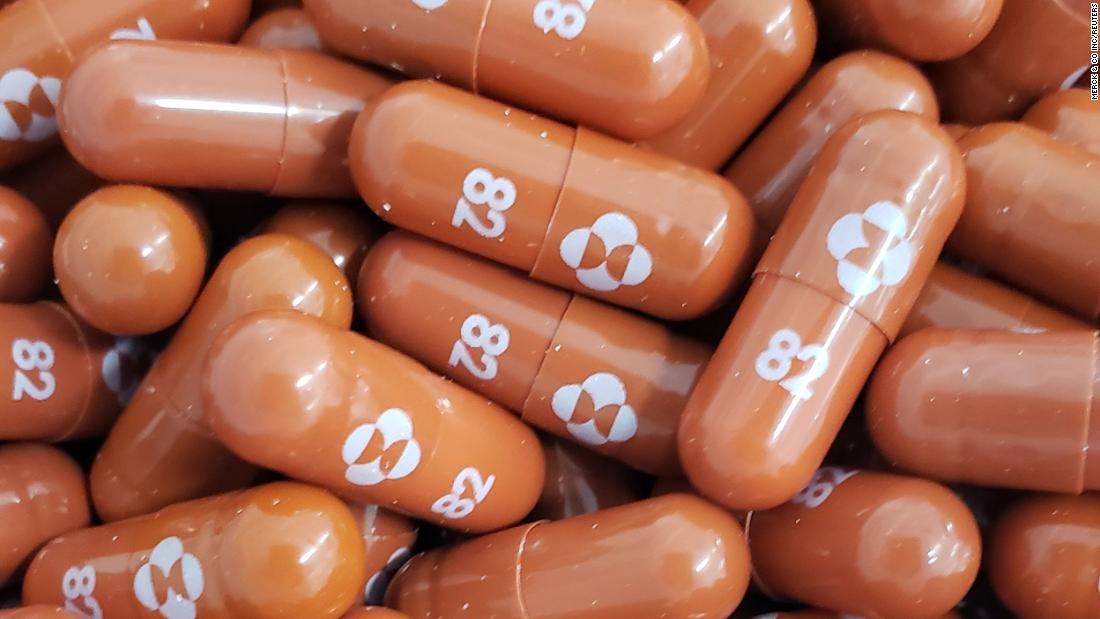
The main competitor is a drug from Merck & Co. and Ridgeback Biotherapeutics called molnupiravir, Dieffenbach said.
This is the product that is being tested at the Kellys trial in Seattle.
Two others are a Pfizer candidate, known as PF-07321332, and AT-527, an antiviral produced by Roche and Atea Pharmaceuticals.
These antivirals work by interfering with the virus's ability to replicate in human cells.
In the case of molnupiravir, the enzyme that copies the viral genetic material is forced to make so many mistakes that the virus cannot reproduce.
That, in turn, lowers the patient's viral load, shortening the time of infection and preventing the kind of dangerous immune response that can cause serious illness or death.
So far, only one antiviral drug, remdesivir, has been approved to treat COVID-19.
But it is given intravenously to patients sick enough to be hospitalized, and it is not intended for early and widespread use.
Instead, the top contenders being studied will be marketed in pill form.
Sheahan, who also did preclinical work on remdesivir, led a first study in mice that showed that molnupiravir could prevent early illness caused by SARS-CoV-2, the virus that causes Covid-19.
The formula was discovered at Emory University and later acquired by Ridgeback and Merck.
Clinical trials have followed, including a first trial with 202 participants last spring that showed molnupiravir rapidly lowered levels of the infectious virus.
Merck CEO Robert Davis said this month that the company expects data from its larger phase 3 trials in the coming weeks, with the possibility of applying for emergency use authorization from the US Food and Drug Administration. (FDA) "before the end of the year."
Pfizer launched a combined phase 2 and 3 trial of its product on September 1, and Atea officials said they expect the results of the phase 2 and 3 trials later this year.
If the results are positive and emergency use is granted for any product, Dieffenbach said, "distribution could start quickly."
That would mean that millions of Americans could soon have access to a daily oral drug, ideally a single pill, which could be taken for five to ten days upon first confirmation of COVID-19 infection.
"When we do, that's the idea," said Dr. Daniel Griffin, an infectious disease and immunology expert at Columbia University.
"Have this all over the country, so people receive it the same day they are diagnosed."
Sotrovimab: this is the treatment with monoclonal antibodies against covid-19
Oral antivirals to treat coronavirus infections, previously marginalized for lack of interest, are now subject to fierce competition and funding. In June, the Biden administration announced that it had agreed to obtain about 1.7 million molnupiravir treatments from Merck, at a cost of $ 1.2 billion, if the product receives emergency authorization or full approval. That same month, the administration said it would invest $ 3.2 billion in the Antiviral Program for Pandemics, which aims to develop antivirals for the covid crisis and others, Dieffenbach said.
The pandemic launched a long-forgotten effort to develop powerful antiviral treatments against coronaviruses, Sheahan said.
Although the original SARS virus in 2003 gave scientists a scare, followed by the Middle East respiratory syndrome, or MERS, in 2012, research efforts slowed with the little persistence of those outbreaks.
"The commercial drive to develop any product was forgotten," Sheahan said.
Widely available antiviral drugs would join monoclonal antibody therapies already used to treat and prevent serious illness and hospitalizations caused by COVID-19.
Monoclonal antibodies produced in the laboratory, which mimic the body's natural response to infection, were easier to develop, but must be administered primarily by intravenous infusions.
The federal government covers the cost of most monoclonal products at $ 2,000 a dose.
It is still too early to know how the price of antivirals might compare.
Like monoclonal antibodies, antiviral pills would not be a substitute for vaccination, Griffin said.
They would be one more tool to fight against covid-19.
"It's good to have another option," he said.
One of the challenges of rapid antiviral drug development is recruiting sufficient numbers of participants for clinical trials, each of which must include hundreds of people, according to Fred Hutch Research Associate Dr. Elizabeth Duke. overseeing the molnupiravir trial.
Participants must be unvaccinated and enroll in the trial within five days of testing positive for COVID.
On any given day, inmates make 100 calls to people who have just tested positive for COVID in the Seattle area, and most say no.
"In general, there is a lot of mistrust in the scientific process," Duke said.
"And some of the people are saying pretty nasty things to the inmates."
Pfizer tests antiviral pill against covid-19 0:34
If antiviral pills are effective, the next challenge will be to establish a distribution system that can get them to people as soon as they test positive.
Griffin said something similar to the program established last year by UnitedHealthcare, which sent Tamiflu kits to 200,000 at-risk patients enrolled in the insurer's Medicare Advantage plans, will be needed.
Merck officials forecast that the company could produce more than 10 million treatments by the end of the year.
Atea and Pfizer have not released similar estimates.
Even more promising?
Studies evaluating whether antivirals can prevent infection after exposure.
"Think about it," said Duke, who is also overseeing a prophylactic trial.
"You could give it to everyone in a home, or everyone in a school. So we are talking about a possible return to a normal life."
- This story was produced by KHN, which publishes California Healthline, an editorially independent service of the California Health Care Foundation
Covid-19