The Centers for Disease Control and Prevention (CDC) authorized the use of the Novavax COVID-19 vaccine on Tuesday, after a panel of experts that advises them recommended it.
The Food and Drug Administration (FDA) approved the vaccine last week, but the CDC needed to issue guidelines on its use so that it could begin to be distributed among the adult population.
The vaccine requires two doses at least 21 days apart in their application (and up to eight weeks later) and
is recommended for the entire population over 18 years of age that has not yet been inoculated
.
3.2 million doses are expected to be available at pharmacies and health care providers in the coming weeks.
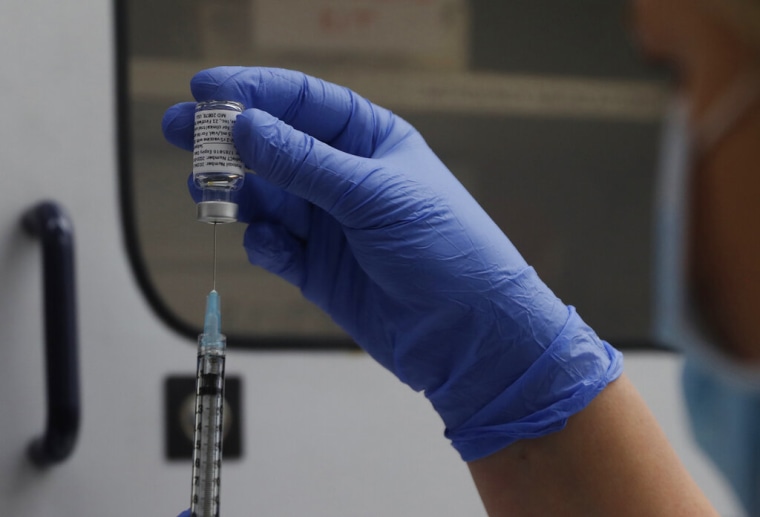
A vial of Novavax's COVID-19 vaccine in this Oct. 7, 2020, file photo. Alastair Grant/AP
Most Americans have already received at least two doses of the COVID-19 vaccines, but CDC officials said there are between 26 million and 37 million adults who have yet to receive any shots.
This is the population that Novavax will primarily target.
"If you have been waiting for a COVID-19 vaccine made with a different technology than what has been available before, now is the time for you to join the millions of Americans who have already been vaccinated," said CDC Director Rochelle Walensky.
[Vaccines against COVID-19 by the nasal route may increase protection. When will they arrive?]
"We really need to focus on that population
," said Dr. Oliver Brooks, a consultant to the CDC and former president of the National Medical Association.
Hopefully, the vaccine will turn them "from unvaccinated to vaccinated."
Novavax is the first protein vaccine to gain approval in the United States.
It is the same technology used by most known vaccines, such as hepatitis B and shingles.
Unlike vaccines from Pfizer and Moderna, which use mRNA technology, Novavax injects copies of the protein spike that are grown in a laboratory and packaged into nanoparticles that resemble a virus to the immune system.
A study reveals that the COVID-19 vaccine can affect menstruation
July 16, 202200:30
Studies with many participants in the United States, Mexico, and Great Britain found that two doses of
the Novavax vaccine were safe and approximately 90% effective in preventing symptomatic COVID-19
.
When the delta variant emerged last summer, Novavax reported that a booster dose revved up virus-fighting antibodies that could target that mutation.
Regarding omicron, Novavax points out that laboratory tests show that the first two injections stimulate the production of antibodies that fight the virus, including against the BA.5 subvariant, which is currently the main threat in the country.
Typical reactions to the vaccines were mild, including arm pain and fatigue, but regulators warned about the possibility of a rare risk, inflammation of the heart, which has also been seen with Pfizer and Moderna vaccines, mainly in teenagers and young men.